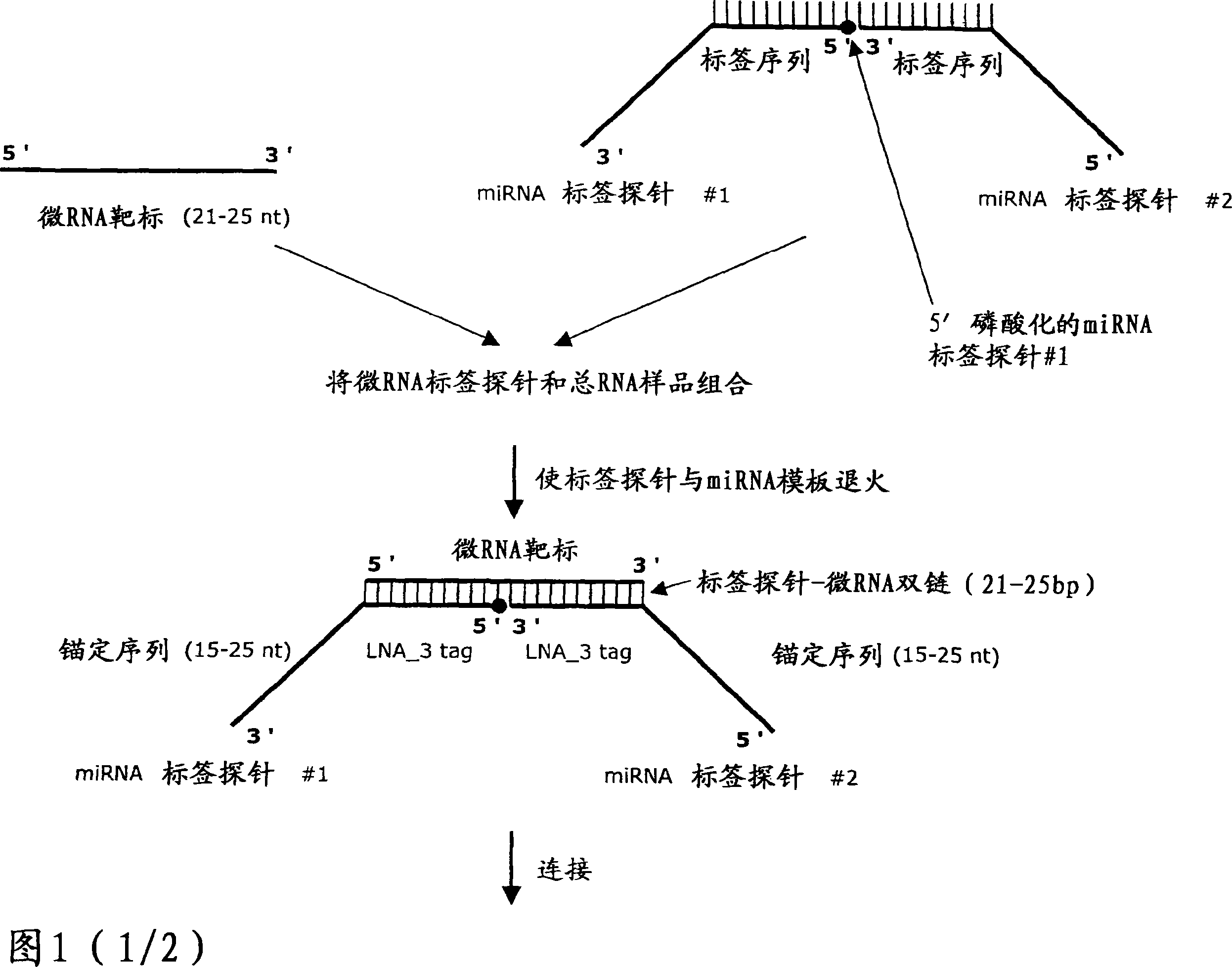
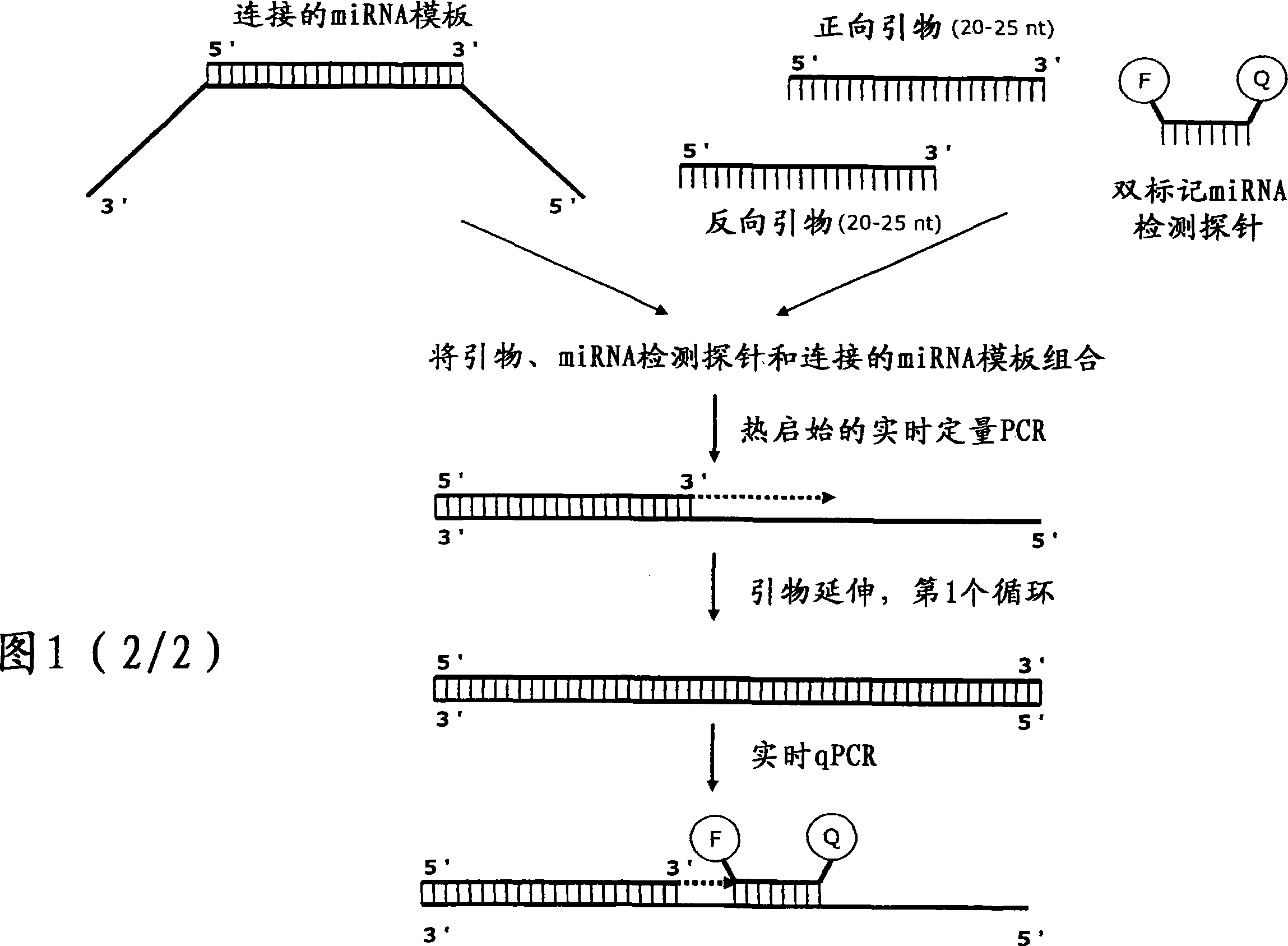
Methods for quantification of microRNA and small interfering RNA
A polymerization, anchoring nucleotide technique, used in the field of detection and quantification of target DNA sequences, which can solve the problem that the factors of rate are poorly understood and none of them are sufficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
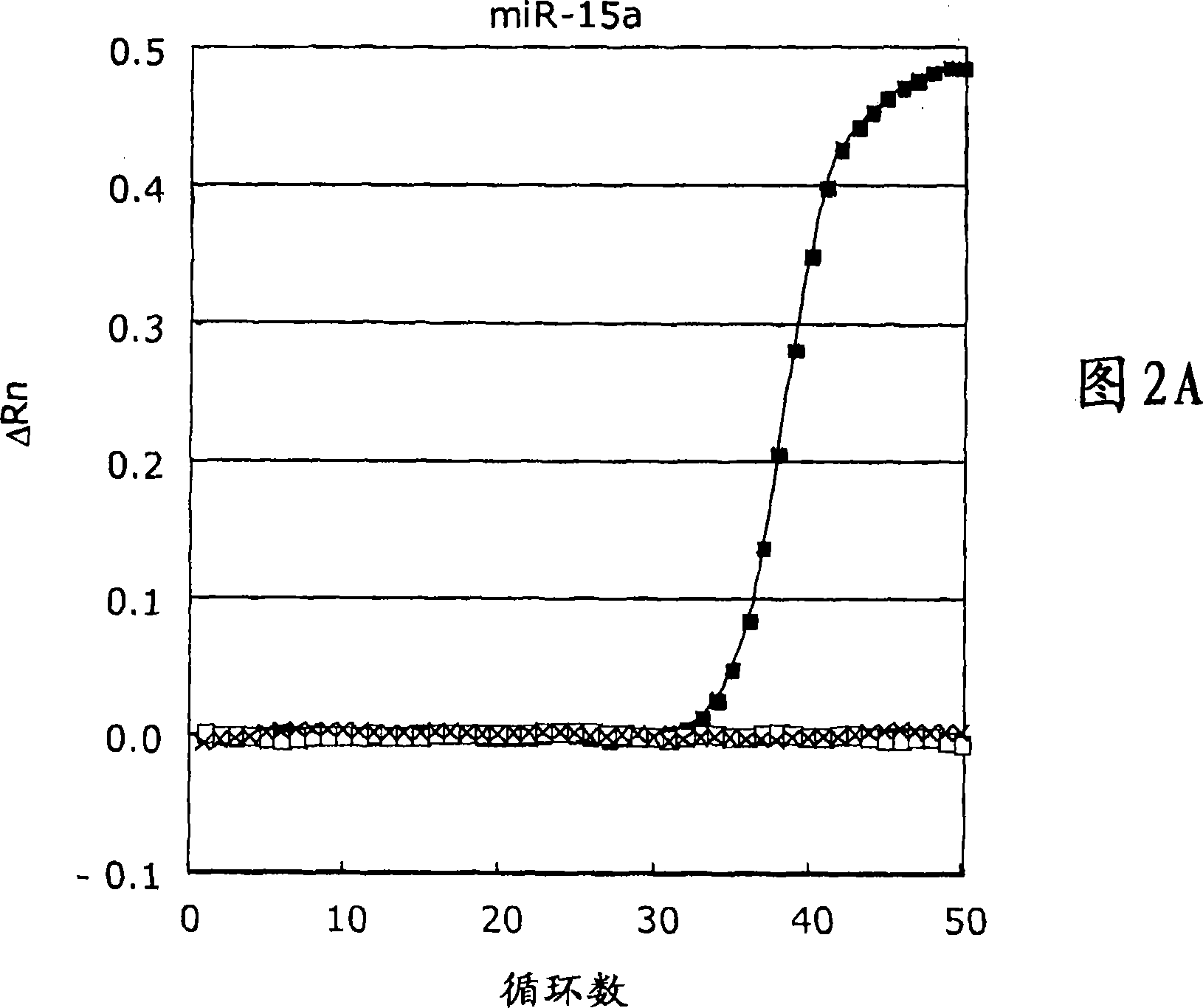
[0331] Real-time quantitative PCR assay for human miR-15a microRNA target sequence
[0332] Sequence-specific LNA-modified microRNA tagging probes are annealed and ligated. The ligated template was subsequently detected with real-time PCR, anchor PCR primers for miR-15a microRNA, and an LNA-modified dual-labeled detection probe, and the negative template was used as a negative control. The specificity of this reaction was tested using a reaction without ligase. The cycle threshold (Ct), which represents the PCR cycle at which the increase in reporter fluorescence above the baseline signal can be detected for the first time, was 35.0 for the attached microRNA probe using the miR-15a microRNA template (Fig. 2A), While no Ct was observed for negative control experiments (negative template and negative ligase, respectively). A normalized reporter signal (Rn), which represents the fluorescent signal of the reporter dye divided by the fluorescent signal of the passive reference dy...
Embodiment 2
[0334] Real-time quantitative PCR assay for human miR-15a microRNA target sequence and corresponding DNA 3'-block target
[0335] The RNA template was replaced with a DNA template chemically blocked with a phosphate at the 3'-end. Without the addition of ligase in the ligation reaction, the blocked DNA template cannot be detected in the LNA sequence-specific real-time PCR assay. The Ct values of RNA template and DNA template were 35.0 and 33.3, respectively (Fig.3).
Embodiment 3
[0337] Specificity of real-time quantitative PCR assays for human miR-15a and human miR-16 microRNA target sequences
[0338] The sequence-specific microRNA target sequence recognition of the present invention was assessed by using the miR-15a microRNA target compared to the human miR-16 target, which has 72% sequence identity to the miR-15a target sequence. Neither the negative template control nor the no template control (NTC) showed any signal in the real-time PCR reaction. Using the hybridization conditions used to anneal the LNA-modified miR-15a target sequence-specific tagging probe to the miR-15a target as described above resulted in a Ct value of 36.2, while using the same tagging probe for the highly homologous The specific miR-16 obtained a Ct value of 39.9, corresponding to a 13-fold discriminative difference (Fig. 4).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com