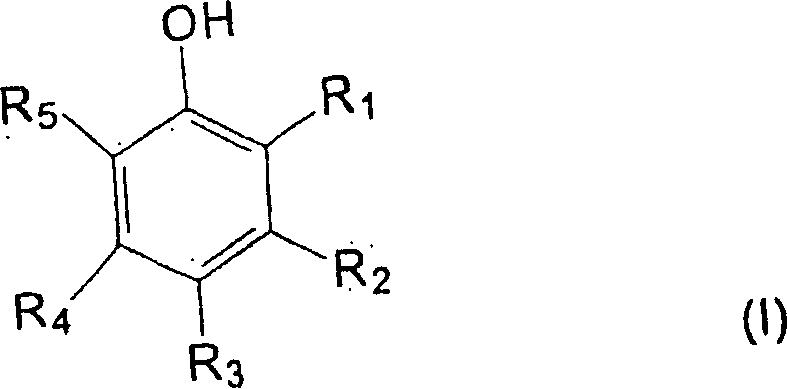
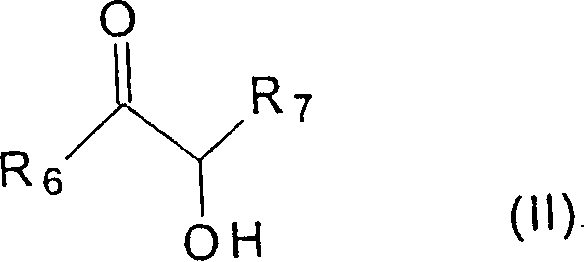
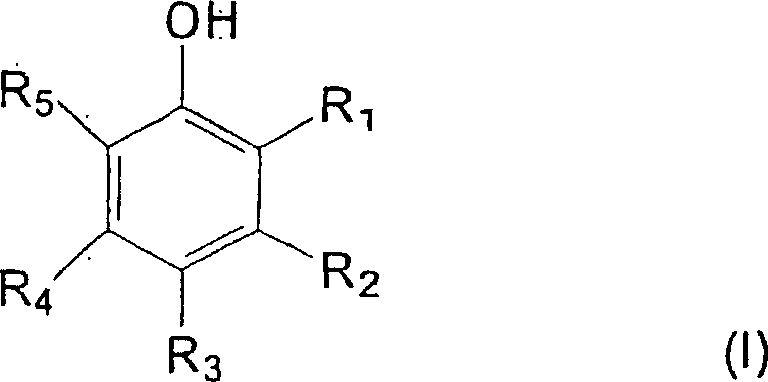
Carboxylic acid modified aminoplast crosslinking agent and powder coating compositions
An aminoplast, crosslinking agent technology, applied in powder coatings, polyester coatings, coatings, etc., can solve problems such as poor powder stability, foaming, and reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The invention also relates to a process for the preparation of the crosslinking agent of the invention. More specifically, the aminoplast resin (a) and the carboxylic acid compound (b) are usually combined with a suitable strong acid as catalyst and optionally a suitable solvent in a suitably equipped reaction vessel. Any suitable solvent may be used, with aromatic solvents being used in most cases. Examples of suitable aromatic solvents include xylene, toluene, and mixtures of these solvents. Examples of strong acids suitable for use as catalysts include, but are not limited to, p-toluenesulfonic acid, dodecylbenzenesulfonic acid, and dodecylbenzenedisulfonic acid. Conventional condensation techniques well known in the art can be used. The reaction mixture is heated to a temperature of 90°C to 160°C, typically 100°C to 140°C, and maintained at that temperature for a time sufficient to obtain an ungelled product having a Tg of at least 10°C. The reaction is terminate...
Embodiment 1
[0061] Into a two-liter four-necked reactor equipped with a thermometer, mechanical stirrer, nitrogen inlet, and by-product (methanol) removal, 640.0 parts by weight of (methoxymethyl)melamine-formaldehyde resin (available from Cytec Industries, Inc.'s CYMEL 303), 488.0 parts by weight of benzoic acid and 1.00 parts by weight of p-toluenesulfonic acid. The mixture was heated to 135°C and maintained at that temperature while methanol by-product was removed from the system. The progress of the reaction was monitored by measuring the IR spectrum and the acid value of the mixture, and the progress of the reaction was terminated when the end point was detected. The final product does not contain any hydroxyl groups and has an acid number of less than about 15 as shown by the IR spectrum. The product obtained was a pale yellow solid with a softening temperature of about 29°C.
Embodiment 2
[0063] To a two liter four-neck reactor equipped with a thermometer, mechanical stirrer, nitrogen inlet, and by-product (methanol) removal, 640.0 parts by weight of CYMEL 303, 610.0 parts by weight of benzoic acid, and 1.20 parts by weight of p-toluenesulfonic acid were added. The mixture was heated to 135°C and maintained at that temperature while methanol by-product was removed from the system. The progress of the reaction was monitored by measuring the IR spectrum and the acid value of the mixture, and the progress of the reaction was terminated when the end point was detected. The final product does not contain any hydroxyl groups and has an acid number of less than about 15 as shown by the IR spectrum. The product obtained was a pale yellow solid with a softening temperature of about 30°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dry film thickness | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
| Softening temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap