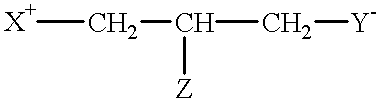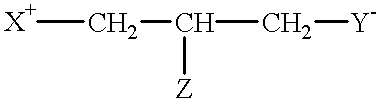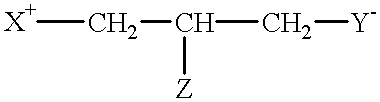Compounds having reversible inhibiting activity of carnitine palmitoyl-transferase
a technology of carnitine palmitoyltransferase and compound, which is applied in the direction of phosphorous compound active ingredients, drug compositions, metabolic disorders, etc., can solve the problem that none of the therapies currently used in the clinic is fully satisfying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
R,S-4-trimethylammonium-3-(nonylcarbamoyl)-aminobutyrate (ST 1251)
Nonyl Isocyanate
[0105] A solution of decanoyl chloride (20 g, 104.8 mmoles) in acetone (30 ml) was dropped into a solution of sodium azide (9.53 g, 146.6 mmoles) in water (30 ml), cooled in an ice bath. The temperature of the azide solution was kept between 10 and 15.degree. C. after one hour, the solution was transferred in a separatory funnel and the lower phase (the aqueous one) was eliminated. The higher phase was transferred into a flask containing 100 ml of toluene, previously warmed at 65.degree. C. After 1.5 hours, the solution was evaporated to dryness, giving 13.37 g crude product, which after vacuum distillation gave 8.3 g pure product in the form of colorless liquid.
[0106] Yield 47%.
[0107] .sup.1H-NMR (300 MHz; CDCl.sub.3):
[0108] .delta.: 3.3 (t, 2H), 1.6 (m, 2H), 1.45-1.2 (m, 12H), 0.9(brt, 3H).
R,S-4-trimethylammonium-3-(nonylcarbamoyl)-aminobutyrate
[0109] Nonyl isocyanate (15.42 g, 91.12 mmoles) was adde...
example 2
R,S-4-quinuclidinium-3-(tetradecyloxycarbonyl)-oxybutyrate (ST 1265)
ter-Butyl R,S-4-guinuclidinium-3-hydroxybutyrate iodide
[0120] Quinuclidine (2.40 g, 21.60 mmoles) was added to ter-Butyl R,S-4-iodo-3-hydroxybutyrate (6.18 g, 21.60mmoles) in acetonitrile (60 ml) and the solution was warmed to 60.degree. C. for 20 hours under stirring. After evaporation of the solvent, the residue was dissolved in acetonitrile and precipitated with ethyl ether several times to give 7.2 g of product, contaminated with about 13% by weight of quinuclidine iodide (as from NMR). After repeated crystallization from CH.sub.3CN / Et.sub.2O, 4.3 g of pure product were obtained.
[0121] Yield 50%.
[0122] M.p.: 124-127.degree. C.
[0123] .sup.1H-NMR (300 MHz; D.sub.2O):
[0124] .delta.: 4.50 (m, 1H), 3.40 (m, 2H), 2.42 (m, 2H), 2.08 (m, 1H), 1.88 (m, 6H), 1.34 (m, 9H).
[0125] FAB Mass=270, [M.sup.+].
[0126] Elemental analysis: responding to the expected formula
[0127] C.sub.15H.sub.28 INO.sub.3.
[0128] K.F.=0.5% water.
[012...
example 3
R,S-4-trimethylammonium-3-(nonylcarbamoyl)-oxybutyrate (ST 1298)
Benzyl ester of R,S-4-trimethylammonium-3-(nonylcarbamoyl)-oxybutyric acid Perchlorate
[0154] Nonyl isocyanate (7.39 g, 43.36 mmoles) was added to a solution of R,S-carnitine perchlorate, benzyl ester (7.69 g, 21.86 mmoles) in toluene (100 ml) and the solution was refluxed for 5 days under stirring. Nonyl isocyanate (1.84 g, 10.86 mmoles) was further added and the reaction mixture was left under reflux for other 5 days. The solvent was vacuum-evaporated and the residue was washed with ethyl ether and subsequently taken up with chloroform, washed with water and dried over anhydrous sodium sulfate. The oil resulting from the evaporation of the organic phase was purified through flash-chromatography column, using a gradient CHCl.sub.3 to CHCl.sub.3: MeOH 95:5. 4.4 g product were obtained in the form of a thick oil.
[0155] Yield 38.6%.
[0156] .sup.1H-NMR (200 MHz; CDCl.sub.3):
[0157] .delta.: 7.3 (s, 5H), 5.4 (m, 2H), 5.05 (m, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com