Bacterial antigens and vaccine compositions
a technology of antigens and compositions, applied in the field of antigenic h . pylori polypeptide, can solve the problems of inadequate or directed spontaneous immune response or other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
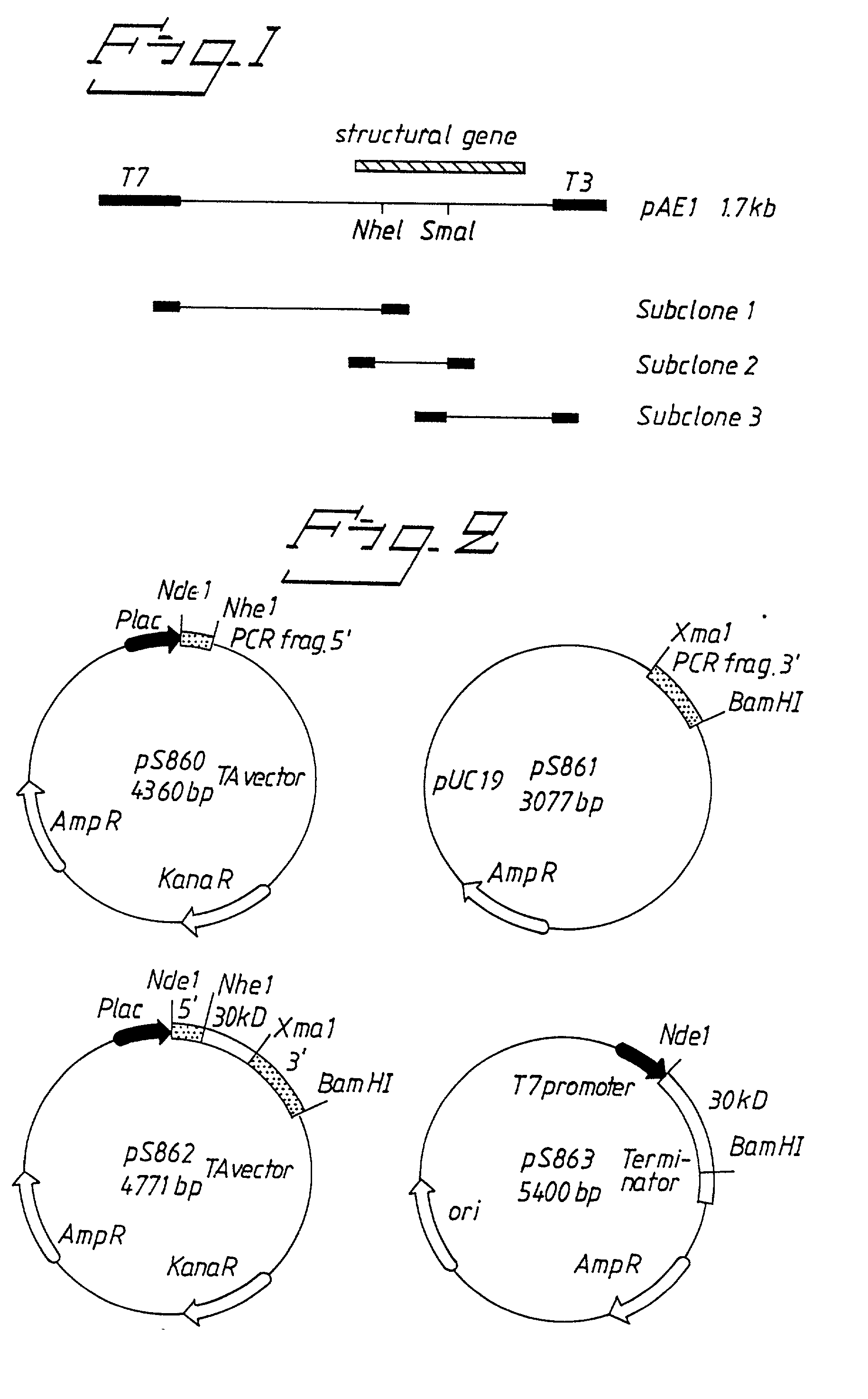
Cloning and Expression of a 29 kDa Polypeptide From H. pylori
1.1. Bacterial Strains, Vectors and Growth Conditions
[0053] H. pylori CCUG 17874 (=NTCC 11637) was grown on horse blood agar plates in an rnicroaerophilic atmosphere. E. coli strains XL1-Blue MRF' and XLOLR (Stratagene, La Jolla, Calif.) were used as host strains for cloning experiments and were grown in Luria-Bertani broth (LB) or NZY medium supplemented with 0.2% maltose and 10 mM MgSO.sub.4 when used for lambda infection. The lambda expression vector ZAP Express.TM. and its phagemid derivative pBK-CMV were obtained from Stratagene.
1.2. DNA Techniques
[0054] Chromosomal DNA from H. pylori 17874 was prepared by suspending bacteria from plates incubated for 48 h in 50 mM Tris-Cl, pH 8.0, 25% sucrose, 50 mM EDTA containing 10 mg / ml lysozyme, and 5 ng / ml DNase-free RNase (Boehringer Mannheim Scandinavia AB, Bromma, Sweden). The suspension was incubated for 10 min at +37.degree. C. An equal volume of lysis buffer (0.4% Triton ...
example 2
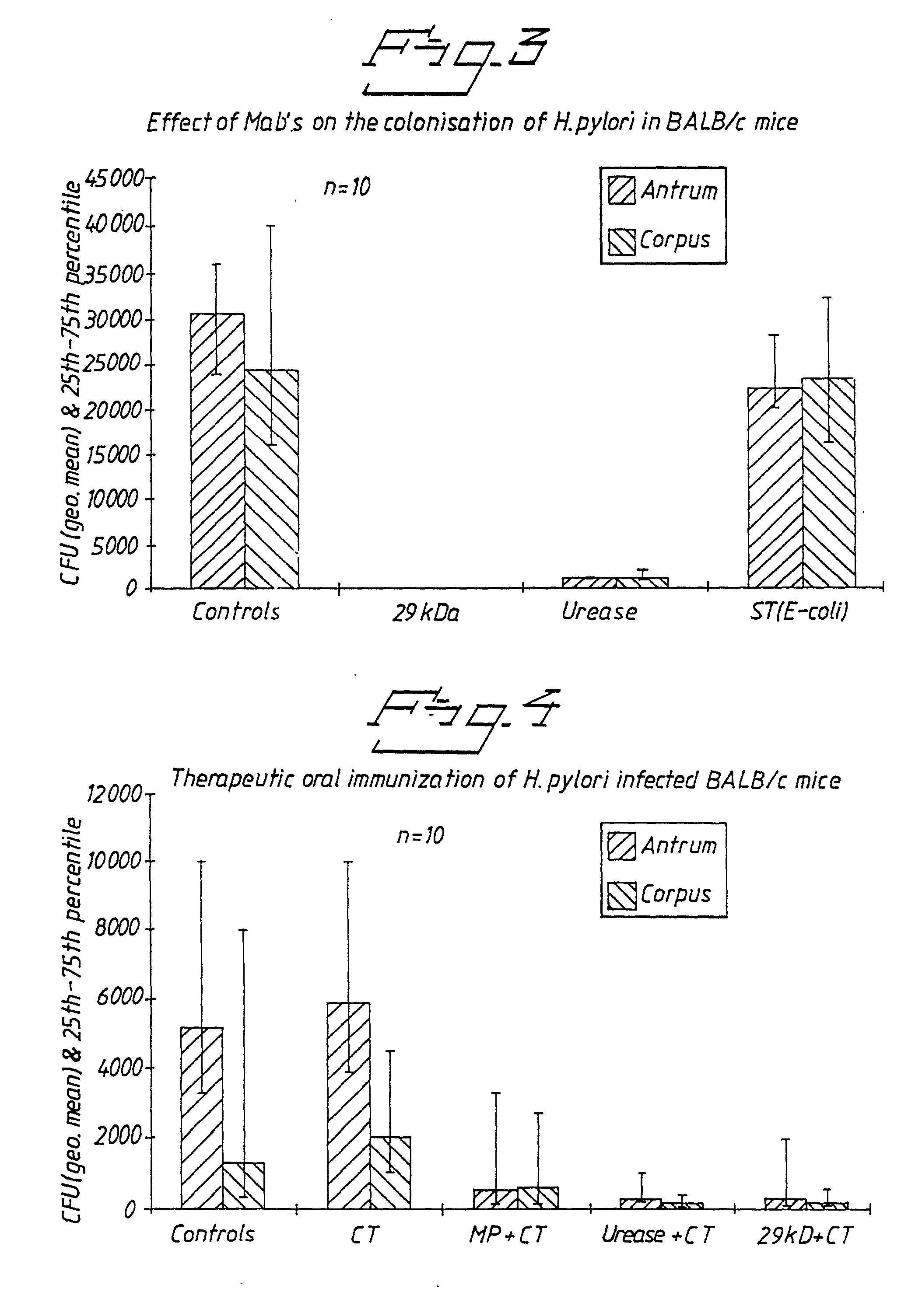
Kinetics of Expression of the 29 kDa Polypeptide during Various Culture Conditions
[0071] Two strains of H. pylori were used, namely CCUG 17874 (a laboratory strain) and Hel 73 (recently isolated from a patient suffering from duodenal ulcer). Cultivation was performed on blood agar plates, as well as in Brucella Broth supplemented with cyclodextrin. All cultures were incubated in a microaerophilic atmosphere consisting of 5% O.sub.2, 10% CO.sub.2 and 85% N.sub.2. Bacteria were harvested after 2, 4 and 7 days, washed once in PBS and kept at -20.degree. C. for subsequent analysis. The expression of the 29 kDa surface polypeptide was analysed by inhibition-ELISA employing specific monoclonal antibodies as previously used for detection of E. coli surface antigens (Lopez-Vidal, Y and Svennerholm, A- M., J. Clin. Microbiol. 28, 1906-) against the polypeptide. These antibodies were also used in immunoelectron microscopy.
[0072] When CCUG 17874 had been cultivated for 7 days, on blood agar pl...
example 3
Antibody Responses Against the 29 kDa Polypeptide
[0073] Antibody responses against the 29 kDa polypeptide were determined in sera and gastric aspirates from patients with duodenal ulcers (n=19), in asymptotic H. pylori carriers (n=18) and in non-infected age-matched controls (n=20).
[0074] Antibody levels against the 29 kDa polypeptide were tested in gastric aspirates and in sera from the three groups of subjects, by means of different ELISA methods. A majority of the infected subjects had significantly higher levels, compared with the healthy control persons, of specific antibodies against the 29 kDa polypeptide both in serum and in gastric aspirates. Antibody titers in asymptotic carriers were comparable to those of the symptomatic patients.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com