Viral vectors having chimeric envelope proteins containing the IgG-binding domain of protein A
a technology of chimeric envelope proteins and viral vectors, applied in the field of viral vectors, can solve the problems of limited application of this approach, no practical general, and possible recombination between the two
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
6.1. Example 1
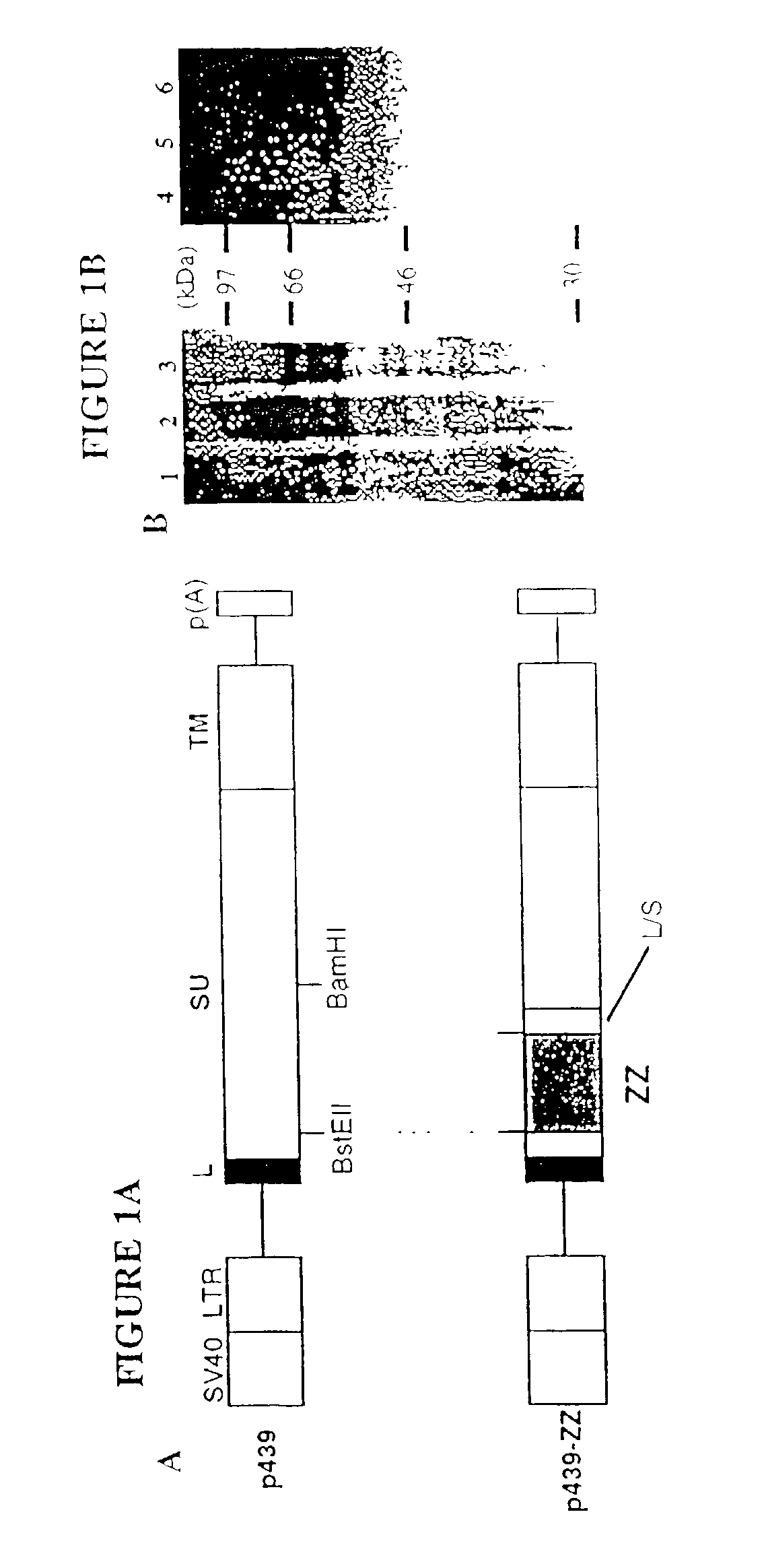
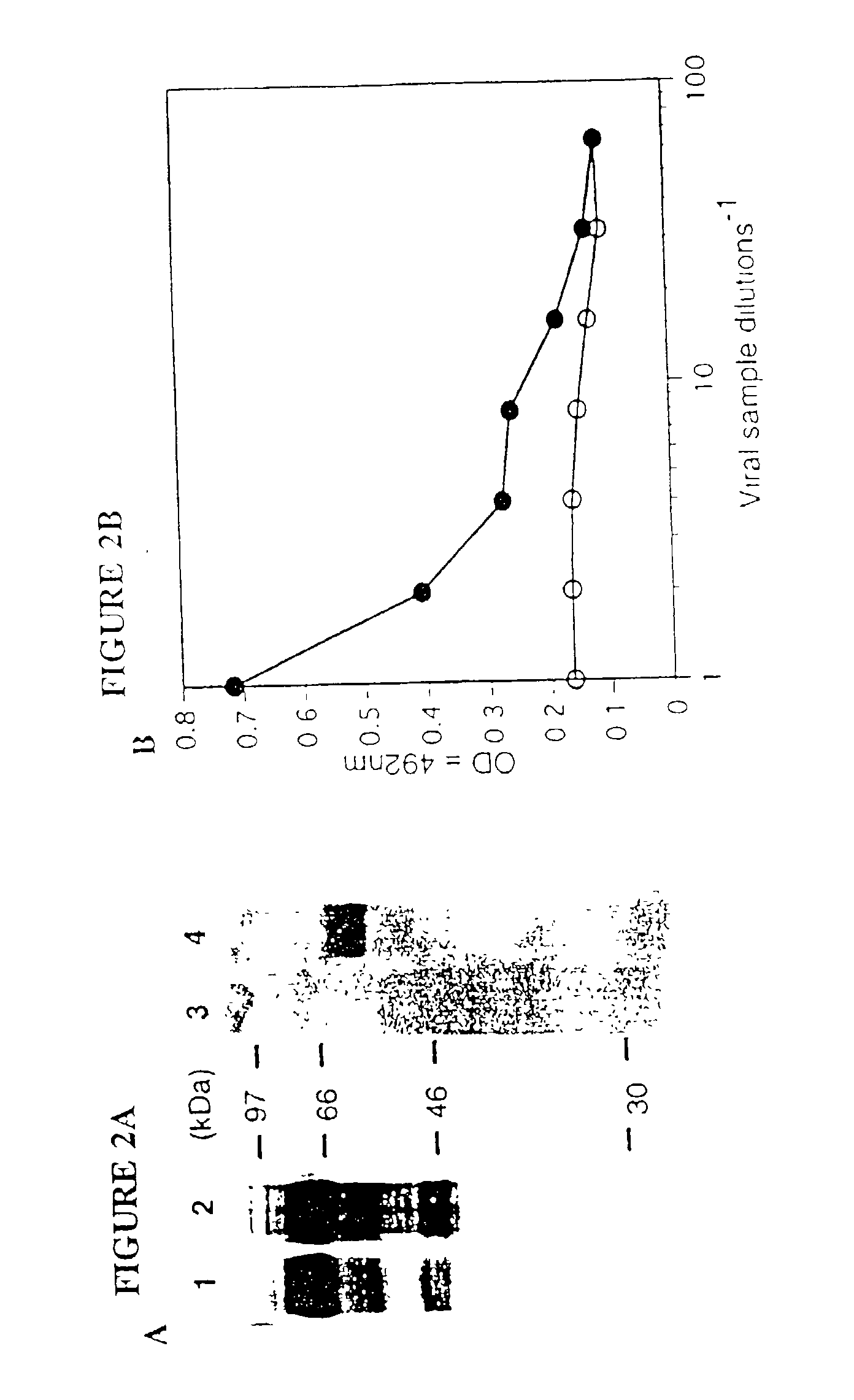
[0040] In this example we describe the construction of a recombinant ecotropic retrovirus displaying protein A-envelope chimeric proteins. Protein A, a protein derived from Staphylococcus aureus, has a strong affinity for the Fc region of various mammalian IgGs (Surolia, A. et al., 1982, Trends Biochem. Sci. 7:74-76). Native protein A has five homologous IgG-binding domains (E, D, A, B and C), and we have utilized the synthetic Z domain which is based on the B domain of protein A (Nilsson, B. et al., 1987, Protein Eng. 1:107-113). The development of retroviral vectors that can bind IgGs (monoclonal antibodies) would have important applications for specific gene delivery.
[0041] Materials and Methods
[0042] 6.1.1. Plasmids and Cell Line.
[0043] A SV40-based plasmid, p439 (SV-E-MLV-env), which express Moloney MLV (Mo-MLV) envelope protein (Landau, N. R. et al., 1992, J. Virol. 66:5110-5113), was kindly provided Dr. Dan R. Littman, New York University. pEZZ 18, which contain...
example 2
6.2. Example 2
[0061] In this example we describe the construction of a recombinant Sindbis virus vector displaying protein A-envelope chimeric proteins to redirect the viral tropism. Protein A (PA), a protein derived from Staphylococcus aureus, has a strong affinity for the Fc region of various mammalian IgGs (Surolia, A. et al., 1982). In contrast to the targeted retroviral vectors described above, the PA-envelope chimeric virus vector once successfully generated needs no further modification to target distinct cells. The targeting is achieved simply by changing the complementary mAb (FIG. 3A). More importantly, we demonstrate that this chimeric virus used in conjunction with mAbs can infect human cells and transfer a test gene, bacterial .beta.-galactosidase with high efficiency. The novel cell targeting system which utilizes PA-mAb interaction for virus infection would have important applications for gene expression studies and therapy.
[0062] 6.2.1. Results
[0063] 6.2.2. Construct...
PUM
| Property | Measurement | Unit |
|---|---|---|
| MW | aaaaa | aaaaa |
| MW | aaaaa | aaaaa |
| MW | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com