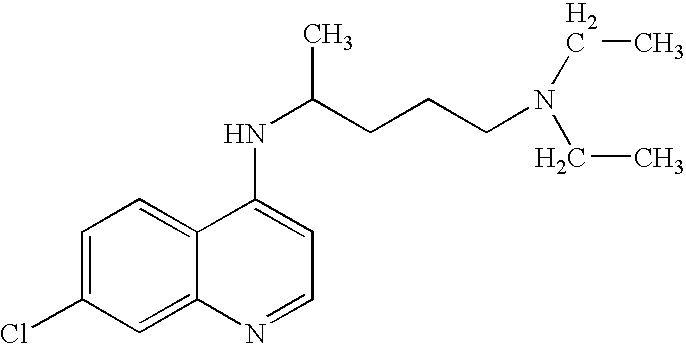
Treatment of DNA damage related disorders
a dna damage and disorder technology, applied in the field of dna damage related disorders, can solve the problems of cancer risk, cancer risk, and subject's caner risk, and achieve the effects of inhibiting cancer invasion, shrinking or eliminating tumors, and reducing or eliminating further growth of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Radioprotection Assay
[0122] HeLa cells were treated with 2 μg / ml of chloroquine for one hour, washed for one hour, and irradiated at 2 or 6 Gy. Subsequently, 1000 cells were plated and assessed for colony formation. Table 2 shows that exposure to chloroquine prior to irradiation increased cell survival by 30%.
TABLE 2TreatmentAverage Number of Colonies*Std. Dev.2 Gy44419.5Chloroquine + 2 Gy58021.26 Gy94.610.6Chloroquine + 6 Gy1298.6
*Averages were from five individual samples.
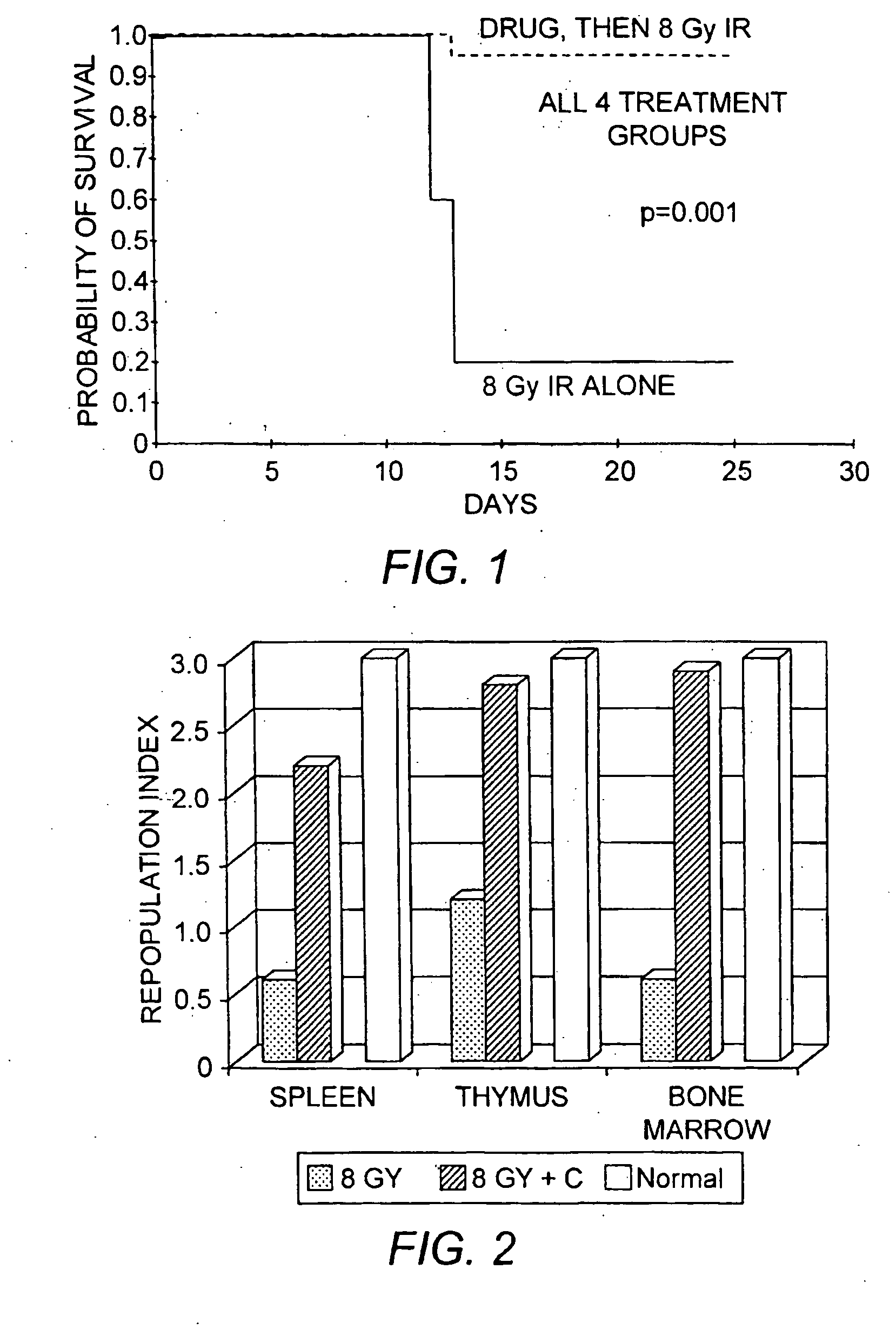
[0123] To test the possibility that chloroquine activation of ATM may cause radioprotection, C57 / BL6 mice were exposed to 8 Gy IR, a dose which kills approximately 80% of the mice at around two weeks. Death appears to result from hematopoietic toxicities. The day before total body irradiation (TBI), mice were either given an i.p. injection of chloroquine or chloroquine was added to the drinking water (5 mice—i.p. 1.75 mg / kg chloroquine; 5 mice—i.p. 3.5 mg / kg chloroquine; 5 mice—1.75 mg / kg chloroquine in drink...
example 2
Cancer Prevention
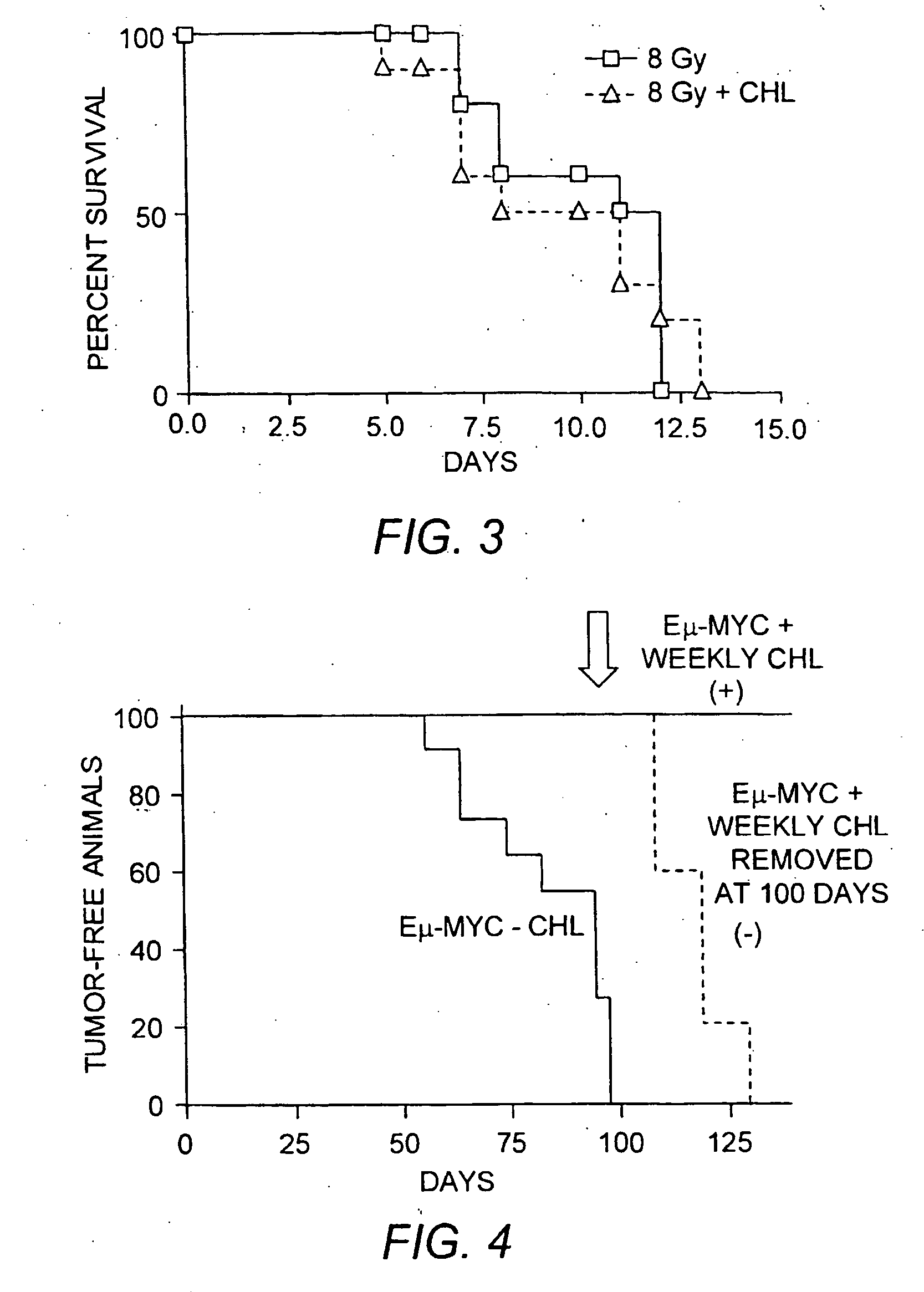
[0125] Transgenic mice expressing the c-myc oncogene under the control of the immunoglobulin enhancer (i.e., E1-myc mice) develop B-cell lymphomas and leukemias with relatively short latencies. Chloroquine was added to the drinking water of a cohort of Eμ-myc mice and the mice were observed for the development of B-cell malignancies. FIG. 4 demonstrates that 100% of the control transgenic mice developed malignancies within 100 days of birth while 0% of the transgenic mice on chloroquine developed tumors. After ˜120 days, half of the cohort of chloroquine-treated mice were taken off of chloroquine and the other half were switched to receiving a dose of chloroquine by i.p. injection once a week. Within ˜30 days, all of the transgenic mice taken off of the chloroquine had developed tumors while none of the mice receiving weekly i.p. injections developed cancer. At 10 months of age, these mice on weekly chloroquine remained cancer-free and appeared healthy and normal. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| multiple-drug resistance | aaaaa | aaaaa |
| drug resistance | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com