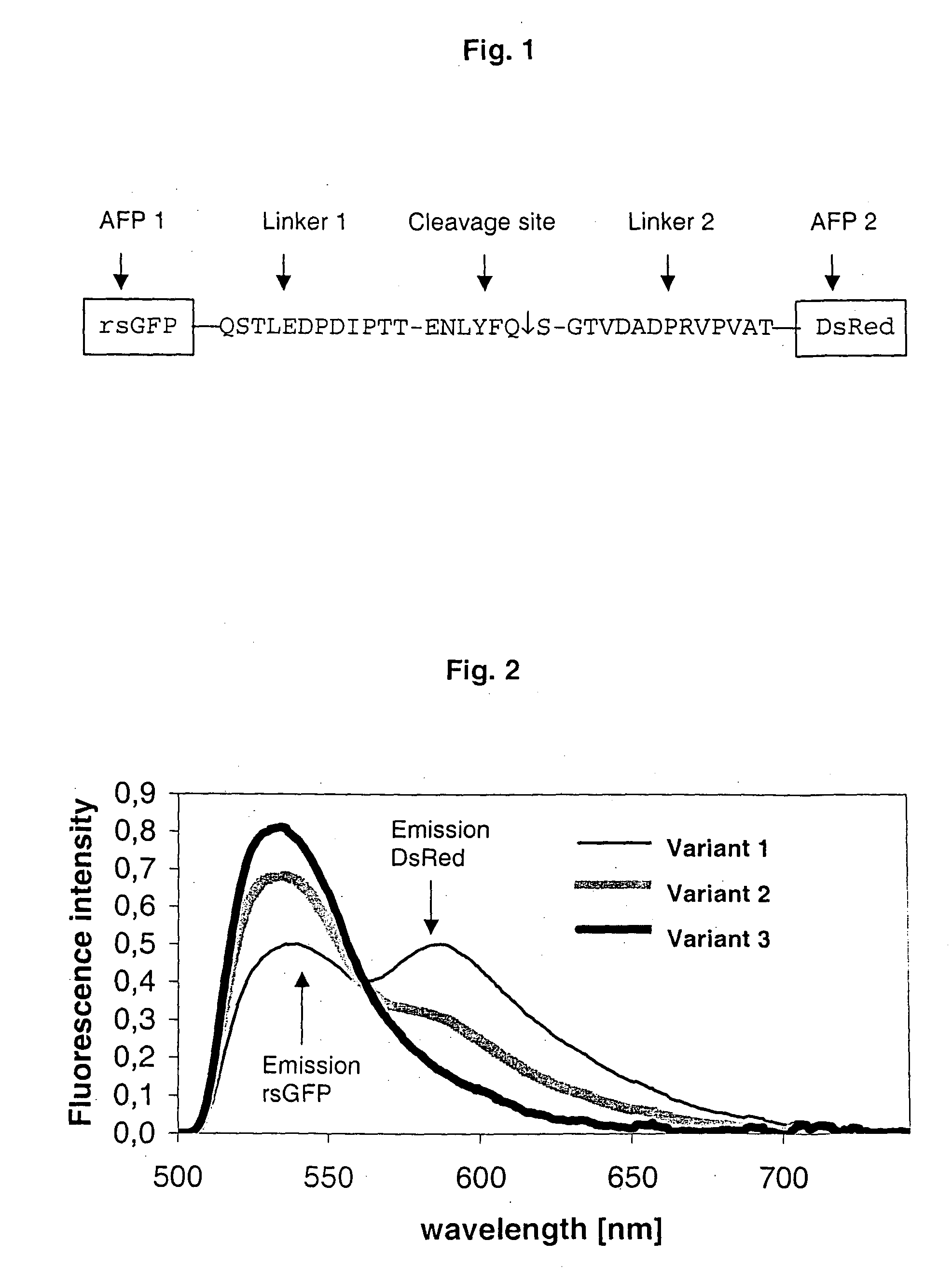
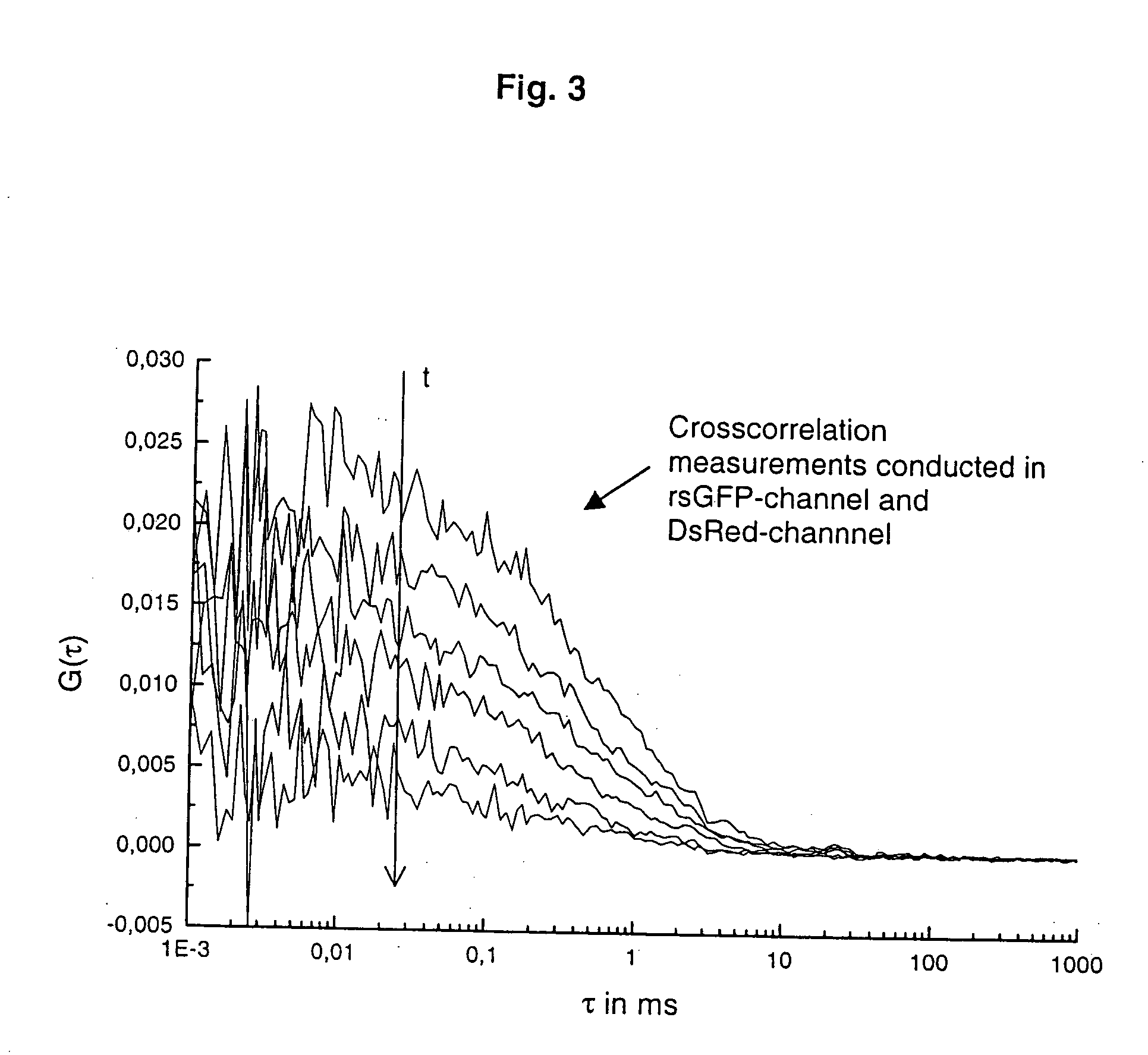
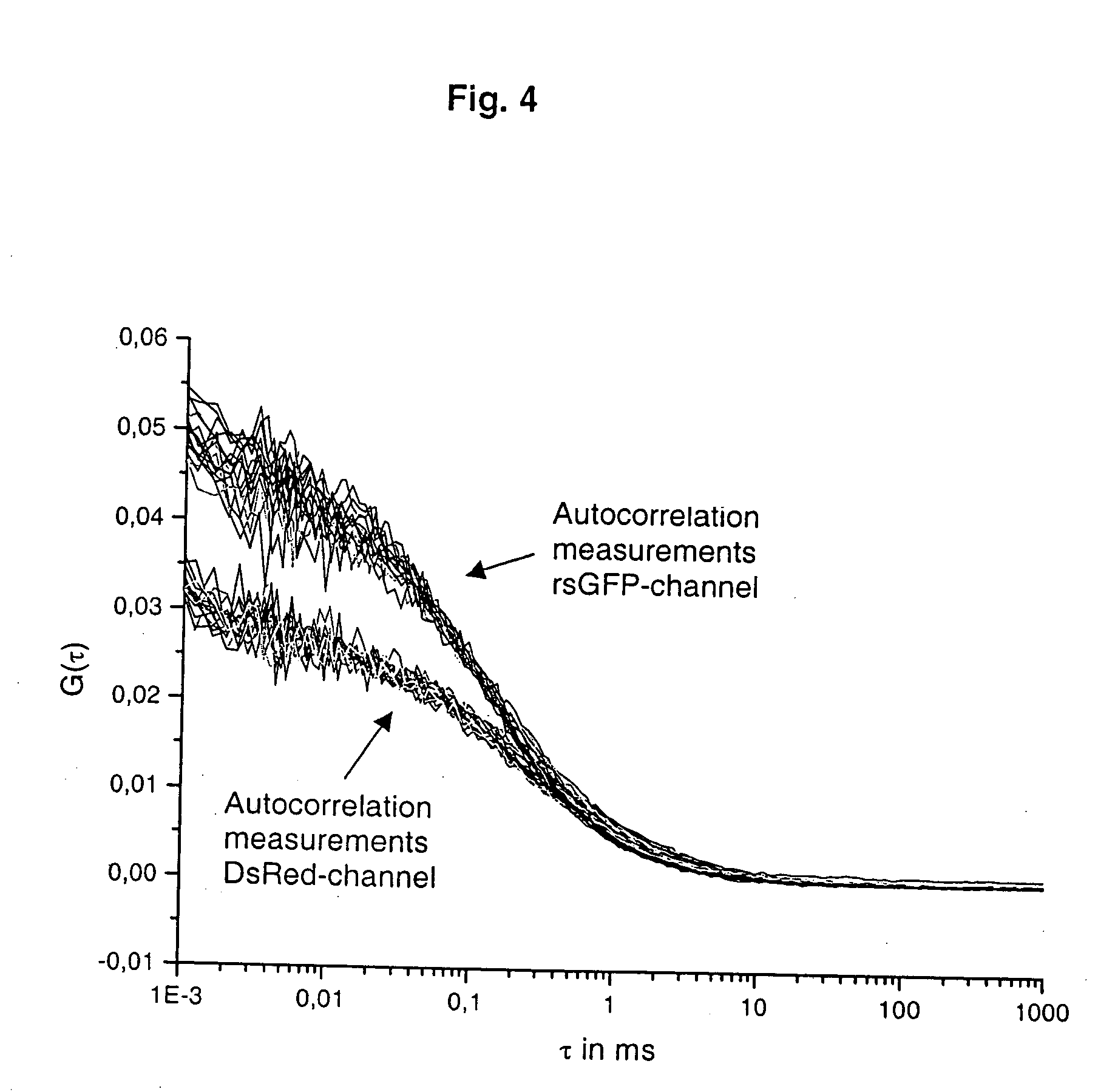
Two coloured fluorimetric protease assay
a fluorimetric protease and colour technology, applied in the direction of fluorescence/phosphorescence, peptides, fungi, etc., can solve the problems of reducing the selectivity and transformation rate of enzymes, limiting their use, and destroying the ability to analyze substrates, etc., to achieve the effect of reducing the cross-correlation amplitud
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Construction and Purification of a Fusion Protein Made from rsGFP and Its Use in an Assay for the TEV Protease
[0062] Strategy of cloning: As minimal quantities of the substance are adequate for the performance of FCS measurements, a vector with lac promoter was used for expression of the fusion proteins, whereby the risk of the formation of inclusion bodies in the bacteria (inclusion bodies) was minimized. To enable an alternative purification with the aid of nickel chelate chromatography, a C-terminal hexahistidine sequence was attached to all of the fusion proteins intended for the expression in E. coli. As the extent to which the amino acid residues in the environment of the protease recognition sequence would affect the reaction was not known, a peptide sequence obtained from the polyprotein of the tobacco etch virus was selected for insertion between the two fluorophores. This sequence is in the region of the cleavage site between capsid protein and polymerase of the virus. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| excitation wavelength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap