Methods for therapy of neurodegenerative disease of the brain
a neurodegenerative disease and brain technology, applied in the field of brain neurodegenerative disease treatment, can solve the problems of affecting the treatment effect, and achieving limited success in direct delivery of neurotrophins through infusion into the neurocompromised brain, and achieves minimal toxicity and high transduction efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Construction of GDNF Expressing Lentiviral Vector
[0053] The cDNA coding for a nuclear-localized β-galactosidase (LacZ) and the human GDNF containing a Kozak consensus sequence (a 636-bp fragment: position 1 to 151 and 1 to 485; GenBank accession numbers L19062 and L19063) were cloned in the SIN-W-PGK transfer vector (R. Zufferey, et al., J. Virol. 73, 2886 (1999), incorporated herein by reference). The packaging construct and vesicular stomatis virus G protein (VSV-G) envelope used in this study were the PCMVDR8.92, PRSV-Rev, and the PMD.6 plasmids described previously (see, R. Zufferey, D. Nagy, R. J. Mandel, L. Naldini, D. Trono, Nature Biotechnol. 15, 871 (1997); A. F. Hottinger, M. Azzouz, N. Deglon, P. Gebischer, A. D. Zum, J. Neurosci. 20, 5587 (2000), incorporated herein by reference). The viral particles were produced in 293T cells as previously described.
[0054] The titers (3 to 5×108 TU / ml) of the concentrated LacZ-expressing viruses (200,000 and 250,000 ng p24 / ml in expe...
example ii
Transgene Expression and Anterograde Transport of GDNF Expression Product Within the Aged Primate Brain
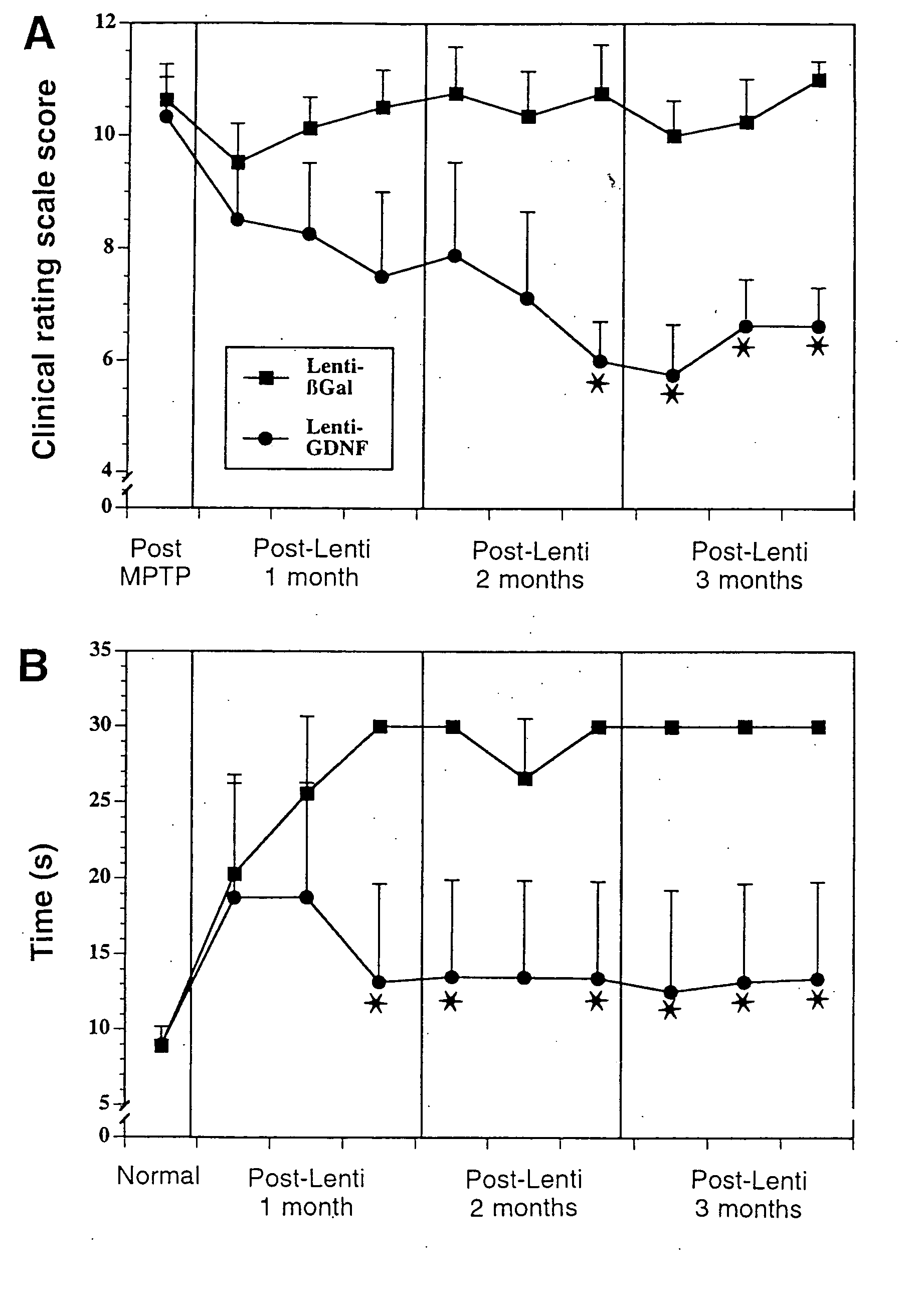
[0055] Non-lesioned aged monkeys that model PD like neurodegeneration (17) display a slow progressive loss of dopamine within the striatum and tyrosine hydroxylase (TH) within the substantia nigra without frank cellular degeneration. Eight aged (approximately 25 years old) female rhesus monkeys received injections of lentiviral vectors encoding—galactosidase (lenti-Gal; n=4) or GDNF (lenti-GDNF; n=4) targeted for the striatum and substantia nigra and were killed 3 months later.
[0056] Under MRI guidance, each monkey received six stereotaxic injections of lenti-Gal or lenti-GDNF bilaterally into the caudate nucleus, putamen, and substantia nigra. Injections were made into the head of the caudate nucleus (10 μl), body of the caudate nucleus (5 μl), anterior putamen (10 μl), commissural putamen (10 μl), postcommissural putamen (5 μl), and substantia nigra (5 μl). Injections were made...
example iii
DOPA Uptake Following GDNF Treatment in Aged Animals
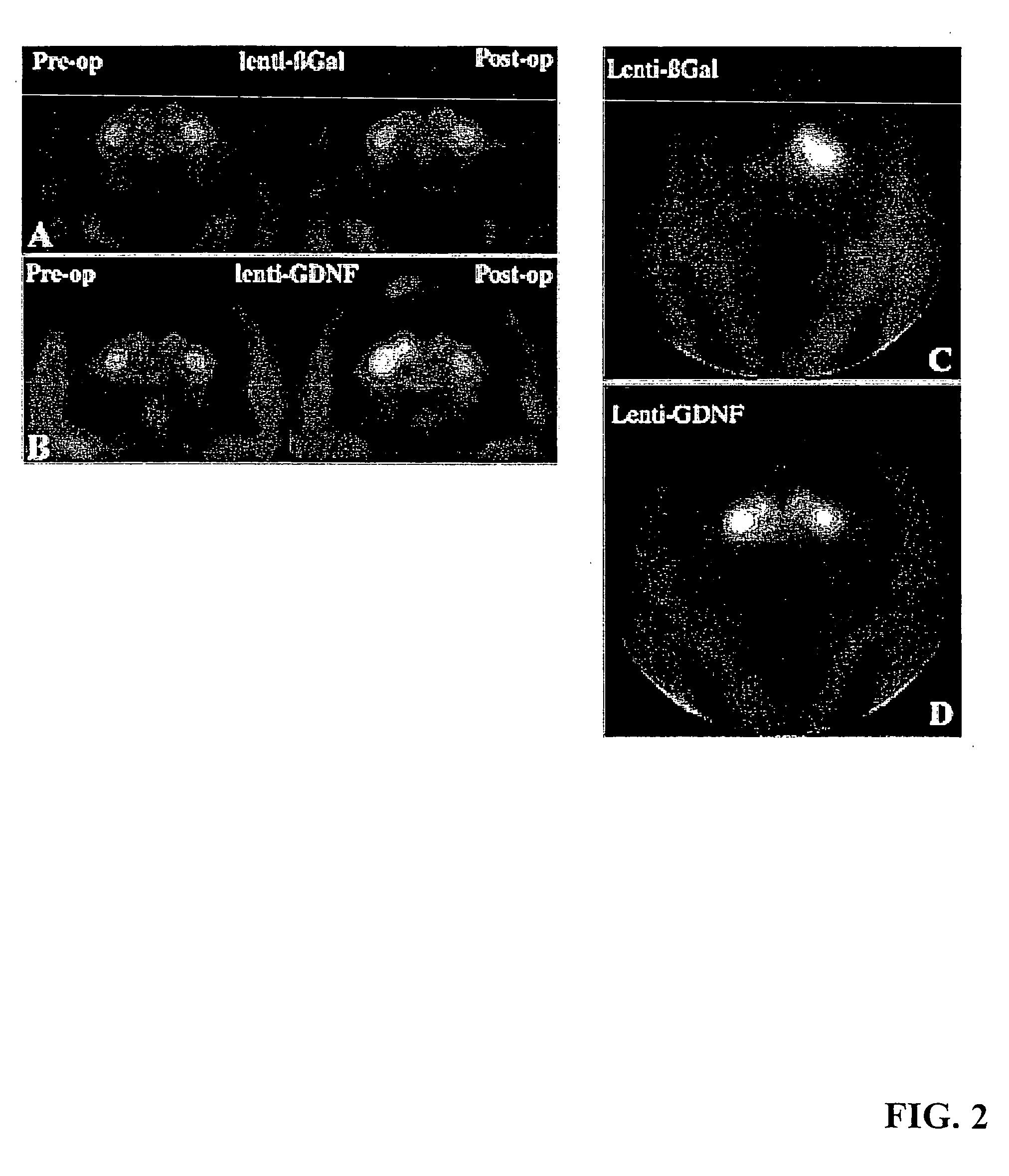
[0061] Aged monkeys underwent fluorodopa (FD) positron emissiontomography (PET) before surgery and again just before being killed. Before treatment, all monkeys displayed symmetrical FD uptake in the caudate and putamen bilaterally (ratio: 1.02±0.02) (FIGS. 2A and 2B, left). Similarly, there was symmetrical (4% difference) FD uptake in all lenti-Gal-treated monkeys after lentivirus injections (FIG. 2A, right).
[0062] In contrast, FD uptake was significantly asymmetrical (27%) in lenti-GDN-F-treated monkeys with greater uptake on the side of the GDNF expression (P<0.007; FIG. 2B, right). With respect to absolute values, lenti-Gal animals displayed a trend toward reduced FD uptake after treatment relative to baseline levels (P=0.06). Qualitatively, three of four lenti-GDNF-treated monkeys displayed clear increases in FD uptake on the treated side.
[0063] Within the striatum, lentiviral delivery of GDNTF increased a number of markers...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com