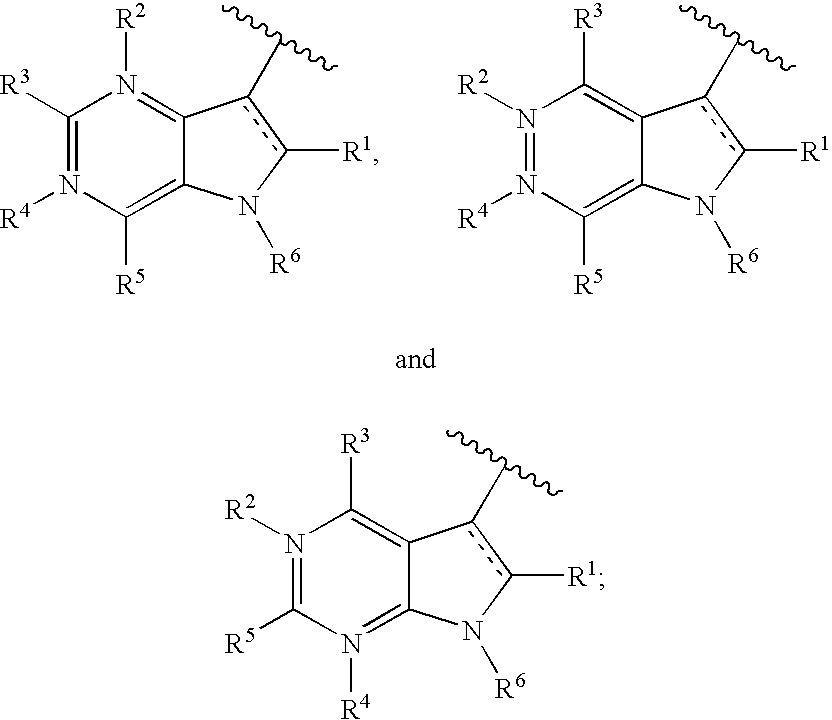
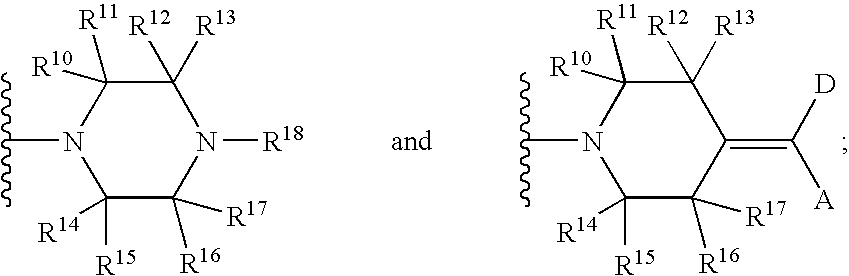Diazaindole-dicarbonyl-piperazinyl antiviral agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation of example 1
2-(4-Benzoyl-piperazin-1-yl)-3-(4-chloro-5H-pyrrolo[3,2-d]pyrimidin-7-yl)-3-oxo-propionitrile (Compound 9)
[0516]
example 1
[0517] To a solution of acid chloride 7 (0.5 mmol) and cyanomethylpiperazine 8 (150 mg, 0.66 mmol) in THF (4 mL) stirring at −35° C. was slowly added a solution of 0.5 M KHMDS in toluene (3.2 mL, 1.6 mmol). The reaction mixture was stirred at −35° C. for 1 h, quenched with sat. aqueous NaHCO3 (50 mL) and extracted with EtOAc (2×50 mL). The combined organics were concentrated and the residue purified by prep HPLC to yield the ketocyano intermediate 9 (39 mg, 0.96 mmol, 19%) as a yellow solid. 1H NMR: (500 MHz, DMSO-d6) δ 8.80 (s, 0.5H), 8.80 (s, 0.5H), 8.65 (s, 0.5H), 8.64 (s, 0.5H), 7.50-7.36 (m, 5H), 4.82-4.77 (m, 1H) 3.80-3.25 (m, 4H) 3.02-2.55 (m, 4H); LC / MS: (ES+) m / z (M+H)+=407; HPLC Rt=0.94 min., column G, conditions B.
preparation of example 2
1-(4-Benzoyl-piperazin-1-yl)-2-(4-chloro-5H-pyrrolo[3,2-d]pyrimidin-7-yl)-ethane-1,2-dione (Compound 10)
[0518]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Antimicrobial properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com