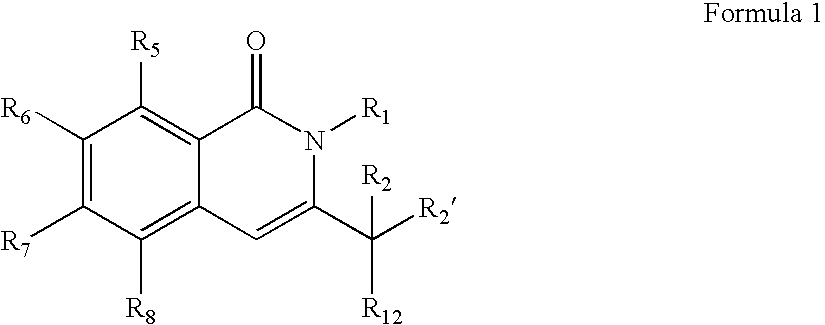
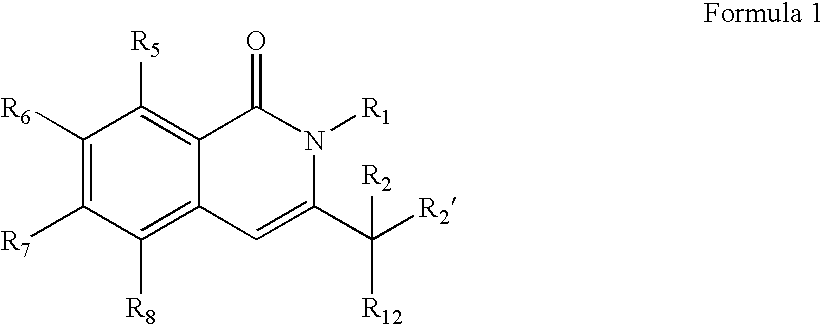
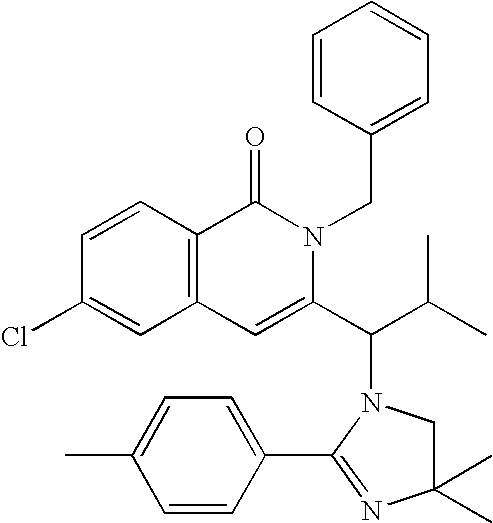
Compounds, compositions, and methods
a technology of compositions and compounds, applied in the field of compositions and methods, can solve the problems of limiting the usefulness of compounds, inducing cancer cell death, and inhibiting cancer cell division
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0344] Synthesis of Compounds
[0345] A solution of 4-chloro-2-iodobenzoic acid 1 (25 g, 88.7 mmol), SOCl2 (100 mL), and DMF (few drops) was warmed gently with a heat gun until the mixture became homogeneous (15 mins). The solution was maintained at 23° C. for an additional 30 mins, then the solution was concentrated. MeOH (200 mL) was added to the crude residue and the solution was maintained at 23° C. for 30 mins. The solution was concentrated and the crude residue was then dissolved in 10:1 hexanes:EtOAc and passed through a plug of silica gel. The eluent was concentrated to provide 26.2 g (100%) of ester 2 as a colorless oil, which solidified upon standing under high vacuum (0.1 Torr).
[0346] A mixture of ester 2 (2.35 g, 7.94 mmol), acetylene 31 (1.72 g, 8.7 mml), Pd(PPh3)Cl2 (140 mg, 0.20 mmol), Cul (19 mg, 0.1 mmol), and TEA (35 mL) was maintained at 50° C. for 1 h. The reaction mixture was diluted with EtOAc (200 mL), washed with water (100 mL), then brine (100 mL). The orga...
example 2
[0353] Synthesis of Compounds
[0354] Isoquinolone 8 (515 mg, 1.47 mmol), aldehyde 12 (255 mg, 1.47 mmol), NaCN(OAc)3BH (420 mg, 1.98 mmol), and CH2Cl2 (4.1 mL) was maintained at 23° C. for 2 h. An additional portion of 12 (225 mg, 1.30 mmol) in CH2Cl2 (0.6 mL) was then added. After an additional 3 h, the reaction mixture was diluted with EtOAc (20 mL) and washed with 1 N NaOH (5 mL) and brine (5 mL). The organic layer was dried (MgSO4), filtered, and concentrated. The resulting residue was purified by flash column chromatography (3:1 hexanes:EtOAc; 1:1 hexanes:EtOAc) to yield 630 mg (86%) of 13.
[0355] To a solution of isoquinolone 13 (85 mg, 0.17 mmol), diisoproylethylamine (DIEA, 0.12 mL, 0.68 mmol), and CH2Cl2 (0.6 mL) at 23° C. was added p-toluoyl chloride (45 μL, 34 mmol). After 4 h, the reaction mixture was diluted with EtOAc (20 mL) and washed with saturated aqueous NaHCO3 (5 mL) and brine (5 mL). The organic layer was dried (MgSO4), filtered, and concentrated. The resulting...
example 3
[0357] Using the methods of the invention as exemplified in Examples 1 and 2 above, the following compounds were prepared:
LRMSStructure(MH) m / z516.2470.2512.2575.8528.0464.0530.0516.2566.2512.2565.8558.2
PUM
| Property | Measurement | Unit |
|---|---|---|
| Configuration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com