Functional fragments of HIV-1 VPR protein and methods of using the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
SUMMARY
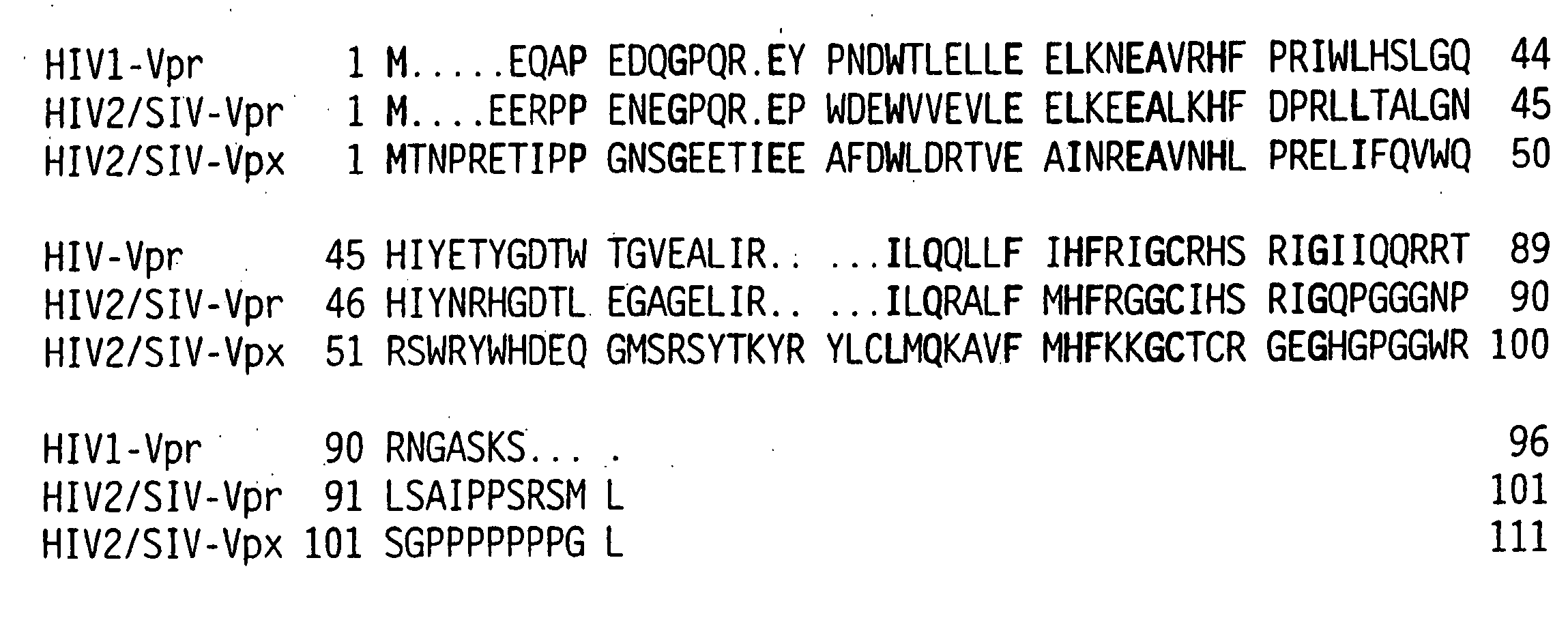
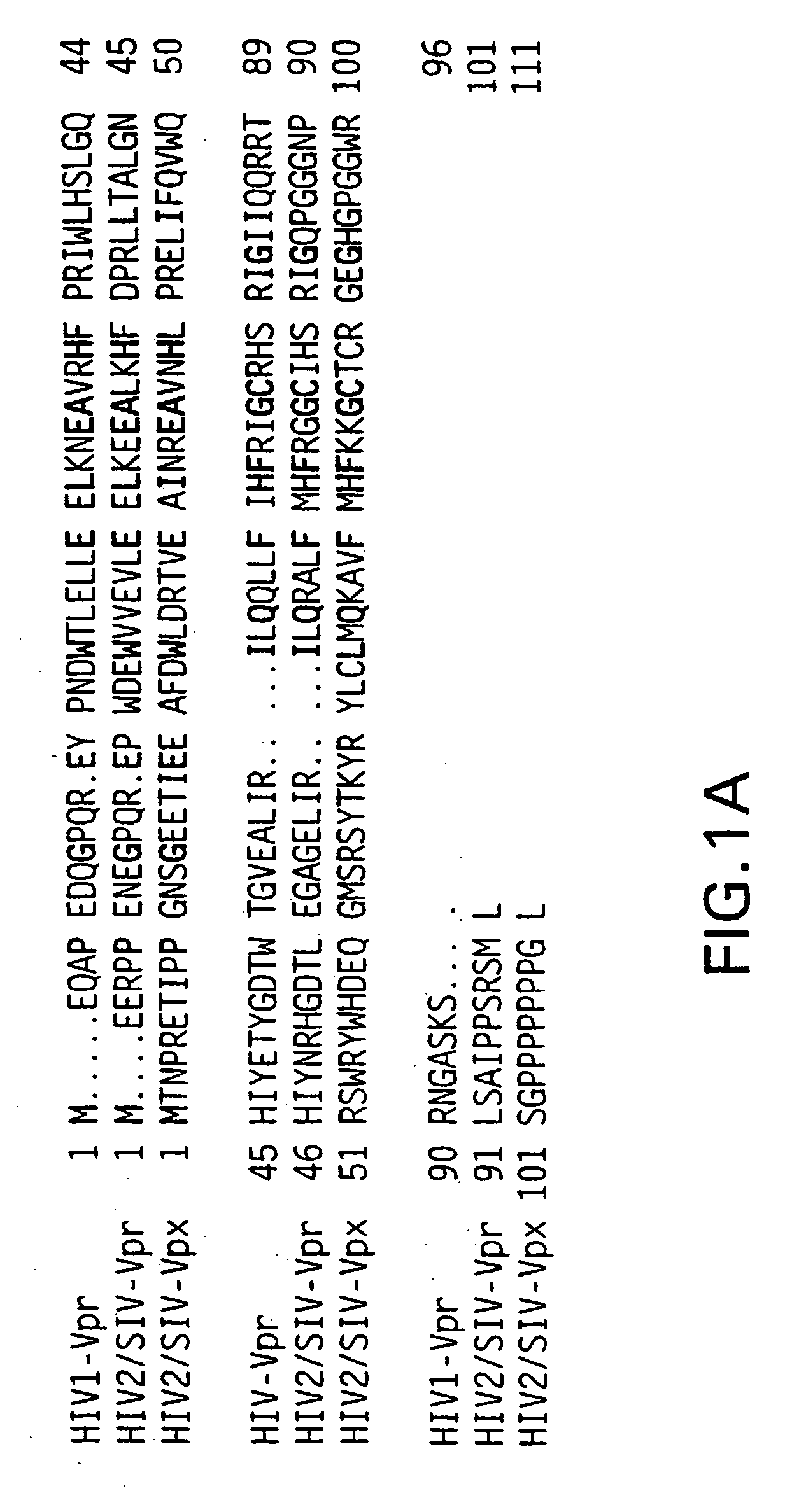
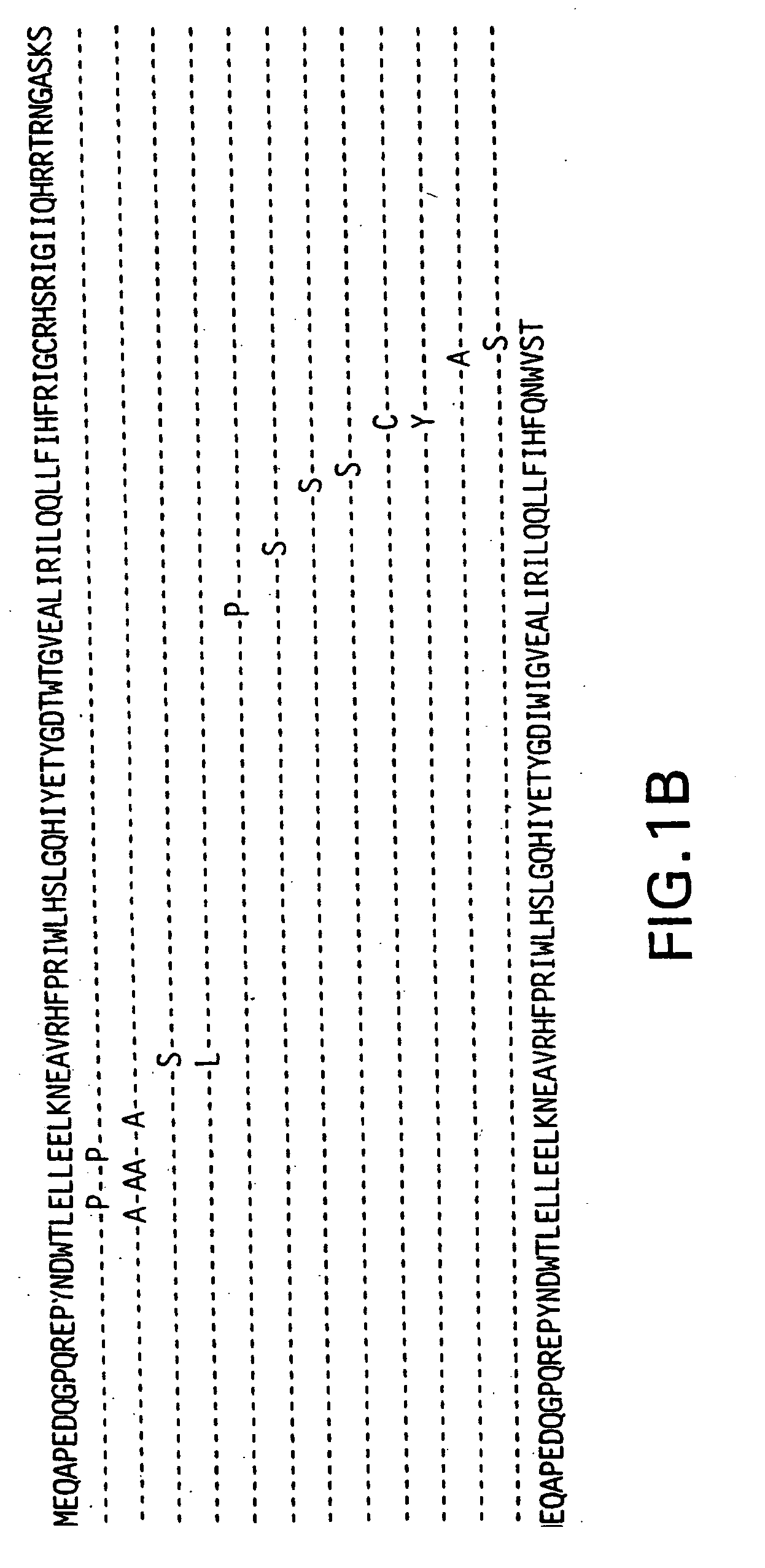
[0101] The vpr gene product of human immunodeficiency virus type 1 (HIV-1) is a virion associated protein that is essential for efficient viral replication in monocyte / macrophages. Vpr is primarily localized in the nucleus when expressed in the absence of other viral proteins. Vpr is packaged efficiently, into viral particles through interactions with the p6 domain of the Gag precursor polyprotein p55gag. We developed a panel of expression vectors encoding Vpr molecules mutated in the amino terminal helical domain, leucine-isoleucine (LR) domain, and carboxy terminal domain to map the different functional domains and to define the interrelationships between virion incorporation, nuclear localization, cell cycle arrest, and differentiation functions of Vpr. We observed that substitution mutations in the N-terminal domain of Vpr impaired both nuclear localization and virion packaging, suggesting that the helical structure may play a vital role in modulating both of these biolo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Antisense | aaaaa | aaaaa |
| Cell proliferation rate | aaaaa | aaaaa |
| Ionicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com