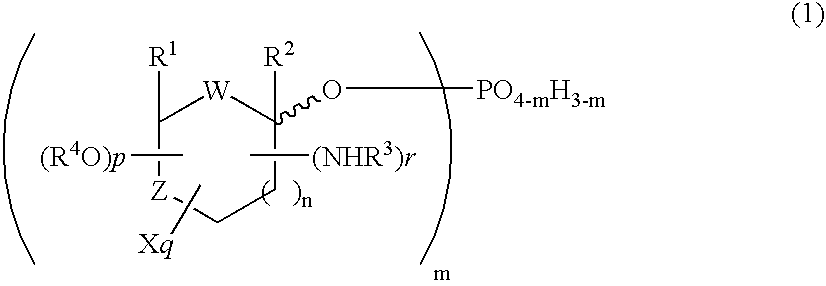
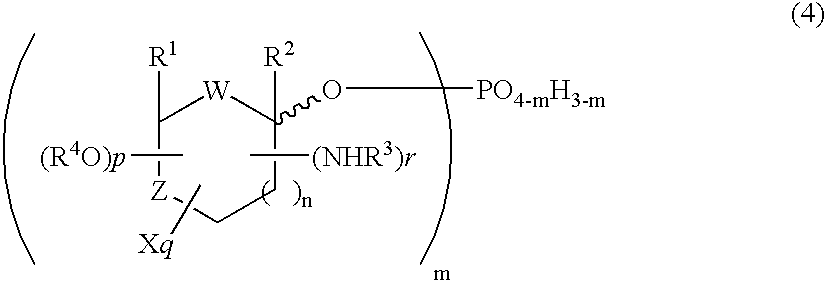
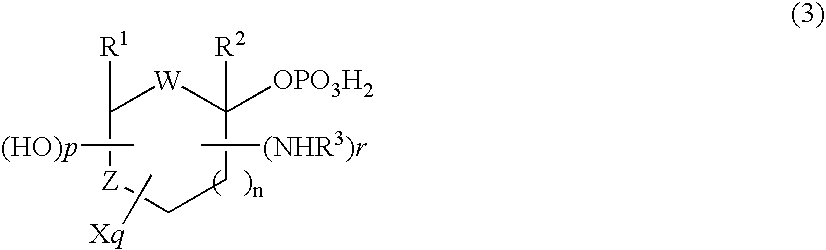Selective process for producing an anomer of a 1-phosphorylated saccharide derivative and process for producing a nucleoside
a technology of saccharide derivative and selective process, which is applied in the direction of sugar derivates, esterified saccharide compounds, organic chemistry, etc., can solve the problems of difficult to establish a general synthetic method for preparing the desired isomer, narrow synthetic process range, poor yield (chem), etc., to achieve good selectivity, improve the inversion rate of the nucleoside, and reduce the cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of an anomer mixture of 3,5-O-bis(4-chlorobenzoyl)-2-deoxy-D-ribose-1-phosphate (18) and bis[3,5-O-bis(4-chlorobenzoyl)-2-deoxy-D-ribos-1-yl]phosphate (19)
[0166] To a mixture of 1.18 g of orthophosphoric acid in 51 mL of acetonitrile were added 2.3 g of tri-n-butylamine and 5.07 g of molecular sieves 4A, and the mixture was cooled to 5° C. with stirring. After one hour, to the mixture was added 5.07 g of 3,5-O-bis(4-chlorobenzoyl)-2-deoxy-α-D-ribosyl chloride (purity: 85%), and the mixture was stirred for one hour to give a solution of a mixture of the title compounds (18) and (19) [(18): (19)=3:5, α-form / β-form of compound (18)=5 / 2] in acetonitrile.
[0167] For preparing a sample for analysis, these compounds were converted into cyclohexylamine salts, which were then purified by silica gel column chromatography to provide two anomer isomers (19a) and (19b) of the title compound (19) from a fraction eluted with methanol-ethyl acetate (1:10).
(19a): Less polar fraction
[...
example 2
Preparation of an anomer mixture of 3,5-O-bis(4-chlorobenzoyl)-2-deoxy-D-ribose-1-phosphate and bis[3,5-O-bis(4-chlorobenzoyl)-2-deoxy-D-ribose-1-yl]phosphate
[0170] To a mixture of 1.11 g of orthophosphoric acid in 49 mL of 2-butanone were added 2.11 g of tri-n-butylamine and 4.9 g of molecular sieves 4A, and the mixture was cooled to 5° C. with stirring. To the mixture was added 4.9 g of 3,5-O-bis(4-chlorobenzoyl)-2-deoxy-α-D-ribosyl chloride (purity: 85%), and the mixture was stirred for 10 min to give a solution of a mixture of the title compounds (18) and (19) [(18) (19)=1:4, α-form / β-form of compound (18)=7 / 10] in 2-butanone.
example 3
Preparation of an anomer mixture of 3,5-O-bis(4-chlorobenzoyl)-2-deoxy-b-ribose-1-phosphate and bis[3,5-O-bis(4-chlorobenzoyl)-2-deoxy-D-ribos-1-yl]phosphate
[0171] To a mixture of 136.8 g of orthophosphoric acid in 2 L of 2-butanone were added 90.6 g of tri-n-butylamine and 200 g of molecular sieves 4A, and the mixture was cooled to 5° C. with stirring. After stirring for one hour, to the mixture was added 200 g of 3,5-O-bis(4-chlorobenzoyl)-2-deoxy-α-D-ribosyl chloride (purity: 85%), and the mixture was stirred for 2 hours to give a solution of a mixture of the title compounds (18) and (19) [(18): (19)=5:4, α-form / β-form of compound (18)=5 / 2] in 2-butanone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com