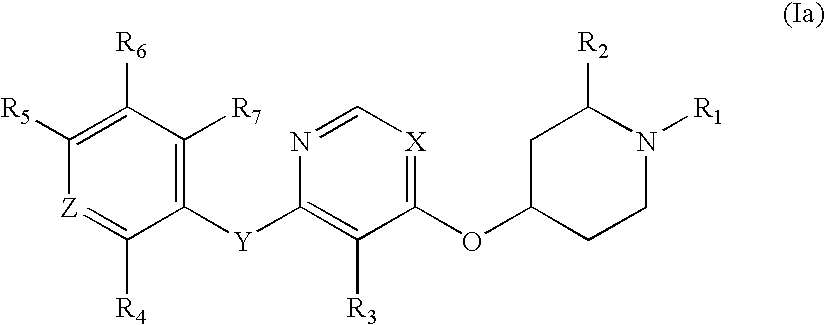
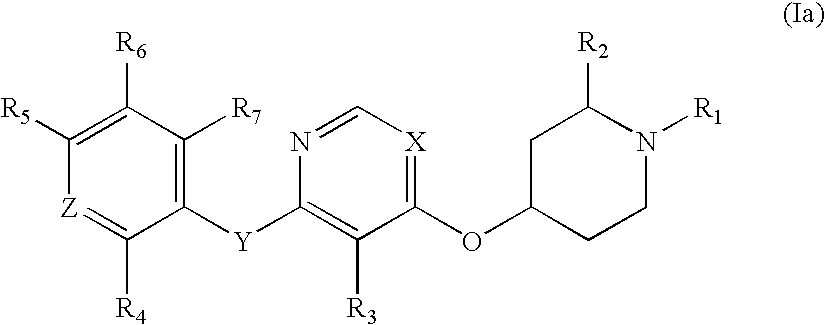
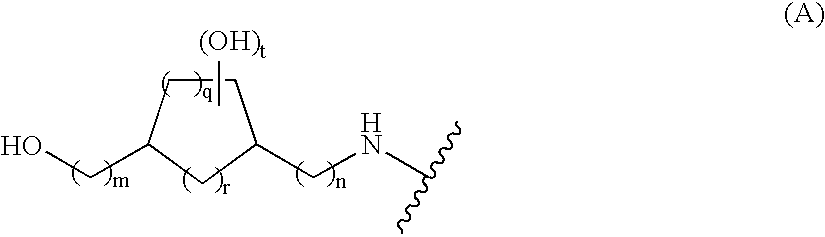
Substituted pyridinyl and pyrimidinyl derivatives as modulators of metabolism and the treatment of disorders related thereto
a technology of pyridinyl and pyrimidinyl derivatives, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, and metabolic disorders, can solve the problems of elevated blood glucose levels, serious health problems, and inability to move glucose into the cells of the individual, and achieve the effect of reducing the weight gain of the individual
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
96-well Cyclic AMP Membrane Assay for RUP3
Materials:
[0358] 1) Adenlyl cyclase Activation Flashplate Assay kit from Perkin Elmer—96 wells (SMP004B) and 125I tracer (NEX130) which comes with the kit. Keep in refrigerator, in a box, and do not expose the Flashplates to light. [0359] 2) Phosphocreatine—Sigma P-7936 [0360] 3) Creatine Phosphokinase—Sigma C-3755 [0361] 4) GTP—Sigma G-8877 [0362] 5) ATP—Sigma A-2383 [0363] 6) IBMX—Sigma 1-7018 [0364] 7) Hepes—1M solution in distilled water—Gibco #15630080 [0365] 8) MgCl2—Sigma M-1028—1M Solution [0366] 9) NaCl—Sigma —S6546—5M Solution [0367] 10) Bradford Protein Assay Kit—Biorad # 5000001 [0368] 11) Proclin 300—Sigma #4-8126
Binding Buffer—filter through 45-micron Nalgene filter and keep in refrigerator. All buffers and membranes should be kept cold (in ice bucket) while performing assay.
20 mM Hepes, pH 7.4
1 mM MgCl2
100 mM NaCl
2× Regeneration Buffer (make in binding buffer):
20 mM Phosphocreatine (1.02 gm / 200 ml binding buffe...
example 2
A. RT-PCR Analysis of RUP3 Expression in Human Tissues (FIG. 1A).
[0409] RT-PCR was applied to determine the tissue distribution of RUP3. Oligonucleotides used for PCR had the following sequences:
ZC47:(SEQ ID NO:3)5′-CATTGCCGGGCTGTGGTTAGTGTC-3′ (forward primer),;ZC48:(SEQ ID NO:4)5′-GGCATAGATGAGTGGGTTGAGCAG-3′ (reverse primer),;
[0410] and the human multiple tissue cDNA panels (MTC, Clontech) were used as templates (1 ng cDNA per PCR amplification). Twenty-two (22) human tissues were analyzed. PCR was performed using Platinum PCR SuperMix (Life Technologies, Inc.; manufacture instructions were followed) in a 50 μl reaction by the following sequences: step 1, 95° C. for 4 min; step 2, 95° C. for 1 min; step 3, 60° C. for 30 sec; step 4, 72° C. for 1 min; and step 5, 72° C. for 7 min. Steps 2 through 4 were repeated 35 times.
[0411] The resulting PCR reactions (15 □l) were loaded on a 1.5% agarose gel to analyze the RT-PCR products, and a specific 466 base-pair DNA fragment represen...
example 3
RUP3 Protein Expression is Restricted to β Cell Lineage of Pancreatic Islets (FIG. 2).
[0418] A. A Polyclonal Anti-RUP3 Antibody was Prepared in Rabbits (FIG. 2A).
[0419] Rabbits were immunized with an antigenic peptide with sequence derived from rat RUP3 (“rRUP3”). The peptide sequence was RGPERTRESAYHIVTISHPELDG (SEQ ID NO: 7) and shared 100% identity with mouse RUP3 in the corresponding region. A cysteine residue was incorporated at the N-terminal end of this antigenic peptide to facilitate KLH crosslinking before injecting into rabbits. The resulting antisera (“anti-rRUP3”) and the corresponding preimmune sera (“pre-rRUP3”) were tested for immune reactivity to mouse RUP3 in immunobloting assays (lanes 1 though 4). In this assay, the GST-RUP3 fusion protein was readily recognized by the anti-rRUP3 antisera (lane 4), but not by the preimmune sera (lane 2). The immunoreactive signal could be efficiently eliminated when the immunobloting assay was performed in the presence of exces...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com