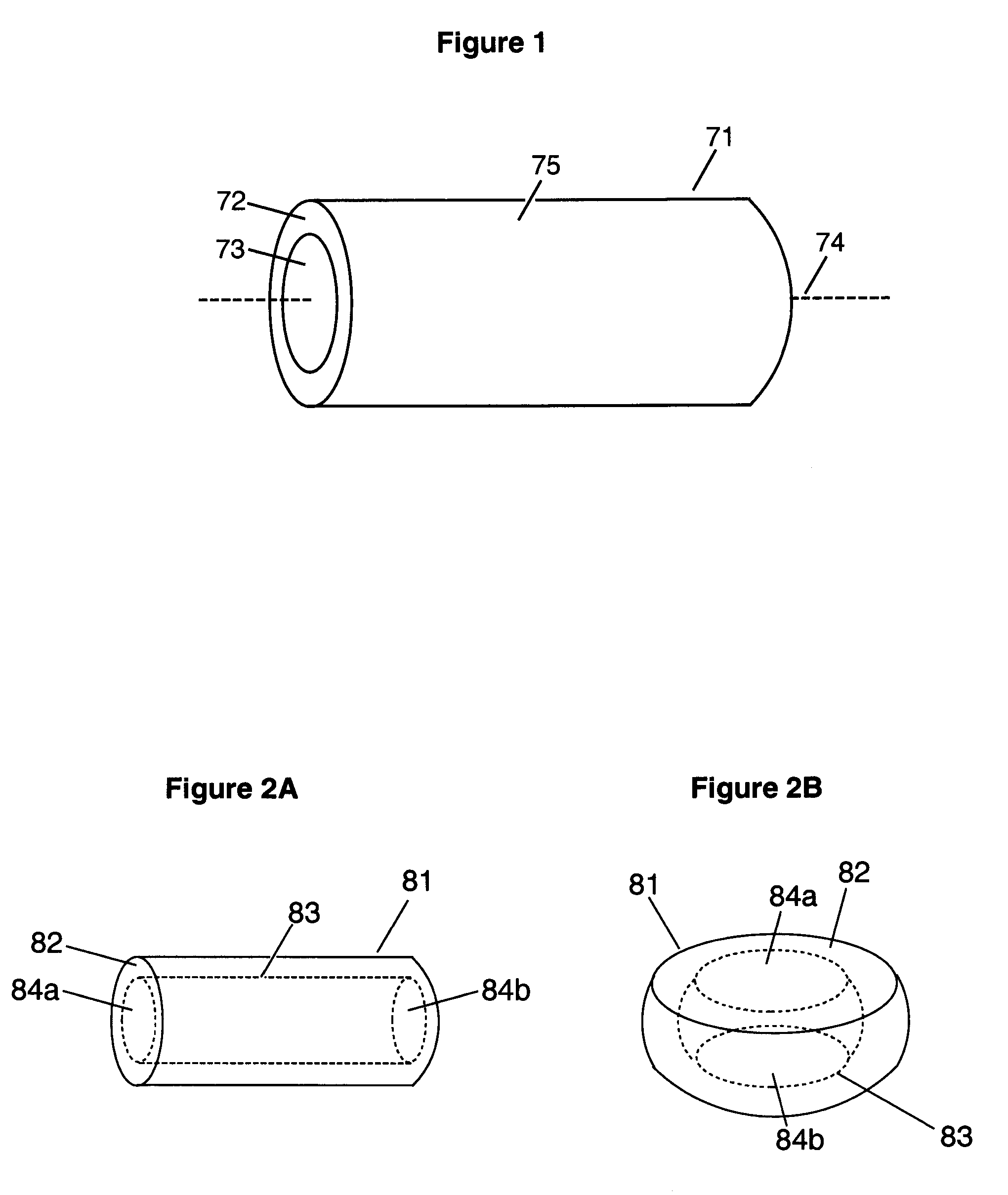
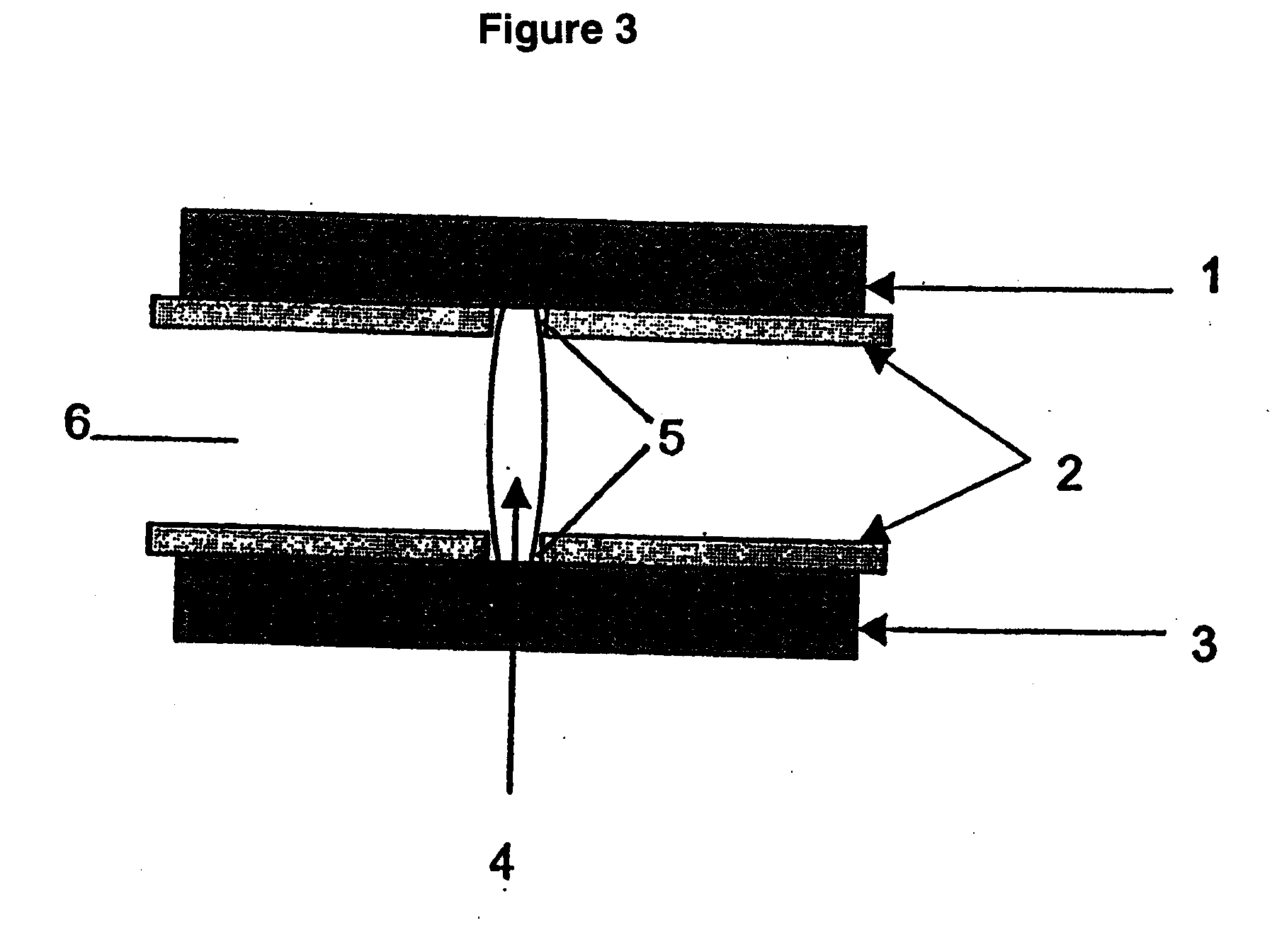
Abuse-proofed dosage form
a dosage form and dosage technology, applied in the field of abuse-proofed dosage forms, can solve the problems of preventing subsequent abuse, pulverizing the dosage form considerably more difficult using conventional means, and preventing the subsequent abuse, so as to prevent parenteral, nasal and/or oral abus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0202]
ComponentsPer tabletPer batchTramadol HCl100.0mg1495.0gPolyethylene oxide, NF, MW 7 000 000167.8mg2508.6g(Polyox WSR 303, Dow Chemicals)Hydroxypropylmethylcellulose33.5mg500.8g100 000 mPa · sPolyethylene glycol (PEG 6000)33.5mg500.8gButylhydroxytoluene (BHT)0.2mg3.0gTotal weight335.0mg5008.2g
[0203] The stated quantity of BHT was dissolved in ethanol (96%), such that a 7.7% (mass / mass) ethanolic solution was obtained. This was mixed initially with 150 g of polyethylene oxide in a high speed mixer for 30 minutes and then the remaining quantity of polyethylene oxide was added and stirring continued for a further 30 minutes. The composition was dried for 12 h at 40° C. All the further components were added and mixed for 15 min in a free-fall mixer. The powder mixture was apportioned into an extruder. Extrusion was performed using a model Micro 27 GL 40 D double screw extruder with a spindle diameter of 18 mm manufactured by Leistritz (Nürnberg). Screws with blunt ends were used, t...
example 2
[0207]
ComponentsPer TabletPer batchOxycodon HCl20.0mg410.1g13.7%Polyethylene oxide 7 000 000107.2mg2199.3g73.2%(Polyox WSR 303, DOW Chemicals)Polyethylene glycol (PEG 6000)15.0mg307.8g10.3%Hypromellose (Metholose 90 SH3.8mg76.8g 2.6%100 000 cP, ShinEtsu)α-Tocopherol0.2mg3.0g 0.1%Aerosil (highly disperse SiO2)0.2mg3.0g 0.1%146.4mg3000.0g 100%
[0208] 50 g of the polyethylene oxide, 3 g α-tocopherol and 3 g Aerosil were mixed to a homogeneous mixture by means of a mortar. All the further components were added and mixed for 15 minutes in a free-fall mixer.
[0209] Extrusion was performed using a model Micro 27 PH 40 D twin screw extruder manufactured by Leistritz (Nürnberg). Screws having eccentric ends were used. The die used is a heatable round die having a diameter of 9 mm.
[0210] The following parameters were selected for extrusion:
Screw speed:100rpmThroughput:4kg / hProduct temperature:134°C.Casing temperature:heating zones 1 to 10:100°C.heating zone 11 (die):120°C.
[0211] The extruda...
example 3
[0215]
ComponentsPer TabletPer batch%Oxycodon HCl20.0mg333.3g11.1Polyethylene oxide 7 000 000122.6mg2060.7g68.7(Polyox WSR 303, DOW Chemicals)Polyethylene glycol (PEG 6000)18.0mg300.0g10.0Hypromellose (Metholose 90 SH18.0mg300.0g10.0100 000 cP, ShinEtsu)α-Tocopherol0.2mg3.0g0.1Aerosil (highly disperse SiO2)0.2mg3.0g0.1180mg3000.0g100%
[0216] 50 g of the polyethylene oxide, 3 g α-tocopherol and 3 g Aerosil were mixed to a homogeneous mixture by means of a mortar. All the further components were added and mixed for 15 minutes in a free-fall mixer.
[0217] Extrusion was performed using a model Micro 27 PH 40 D twin screw extruder manufactured by Leistritz (Nürnberg). Screws having eccentric ends were used. The die used is a heatable round die having a diameter of 9 mm.
[0218] The following parameters were selected for extrusion:
Screw speed:100rpmThroughput:4kg / hProduct temperature:134°C.Casing temperature:heating zones 1 to 10:100°C.heating zone 11 (die):120°C.
[0219] The extrudate, whic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com