1,1,1,3,3,3-hexafluoropropane purification with photochlorination equipment
a technology of photochlorination equipment and hexafluoropropane, which is applied in the direction of halogenated hydrocarbon preparation, halogenated hydrocarbon separation/purification, organic chemistry, etc., can solve the problems of unsatisfactory effects on the environment, and troublesome impurities in certain fluorocarbons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
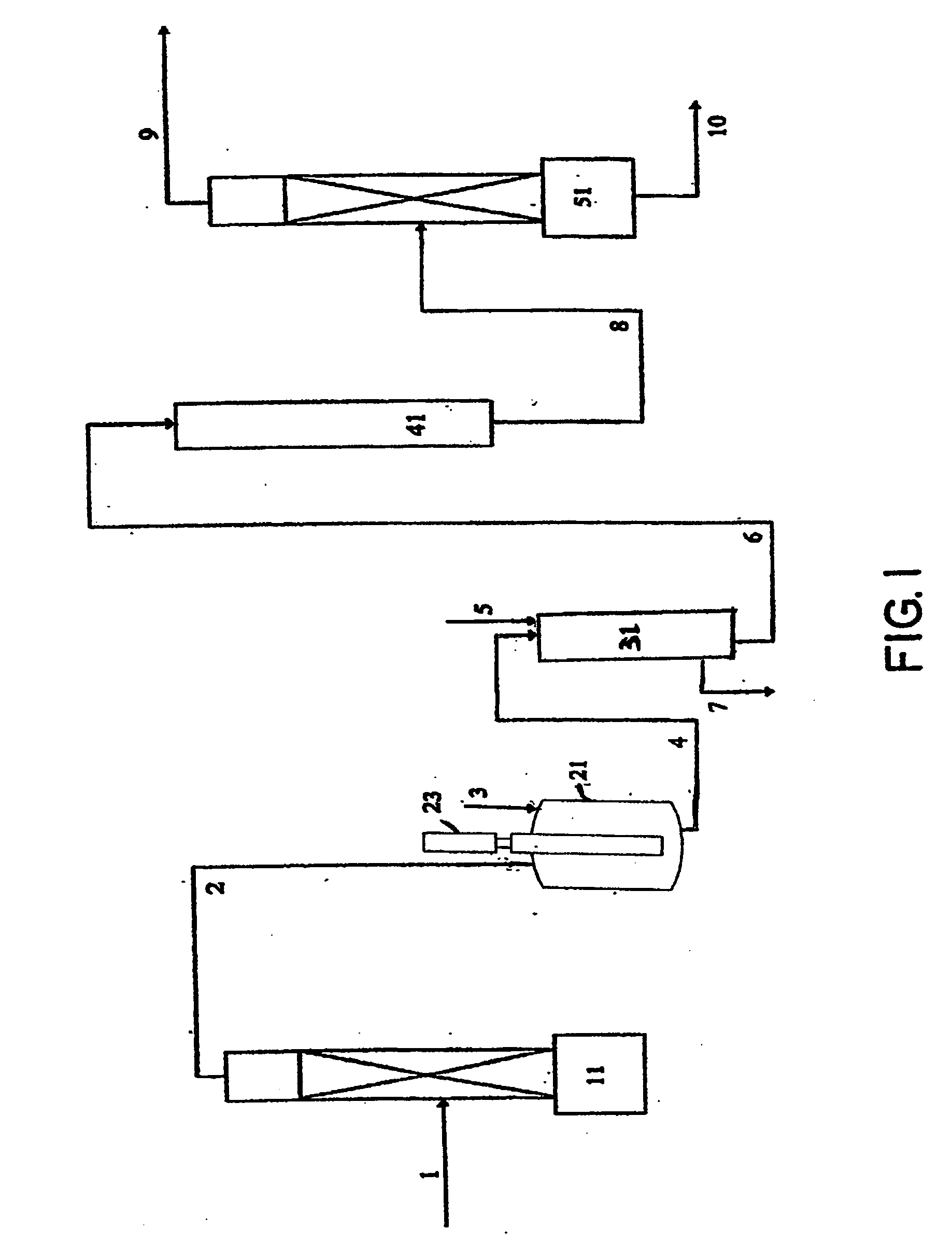
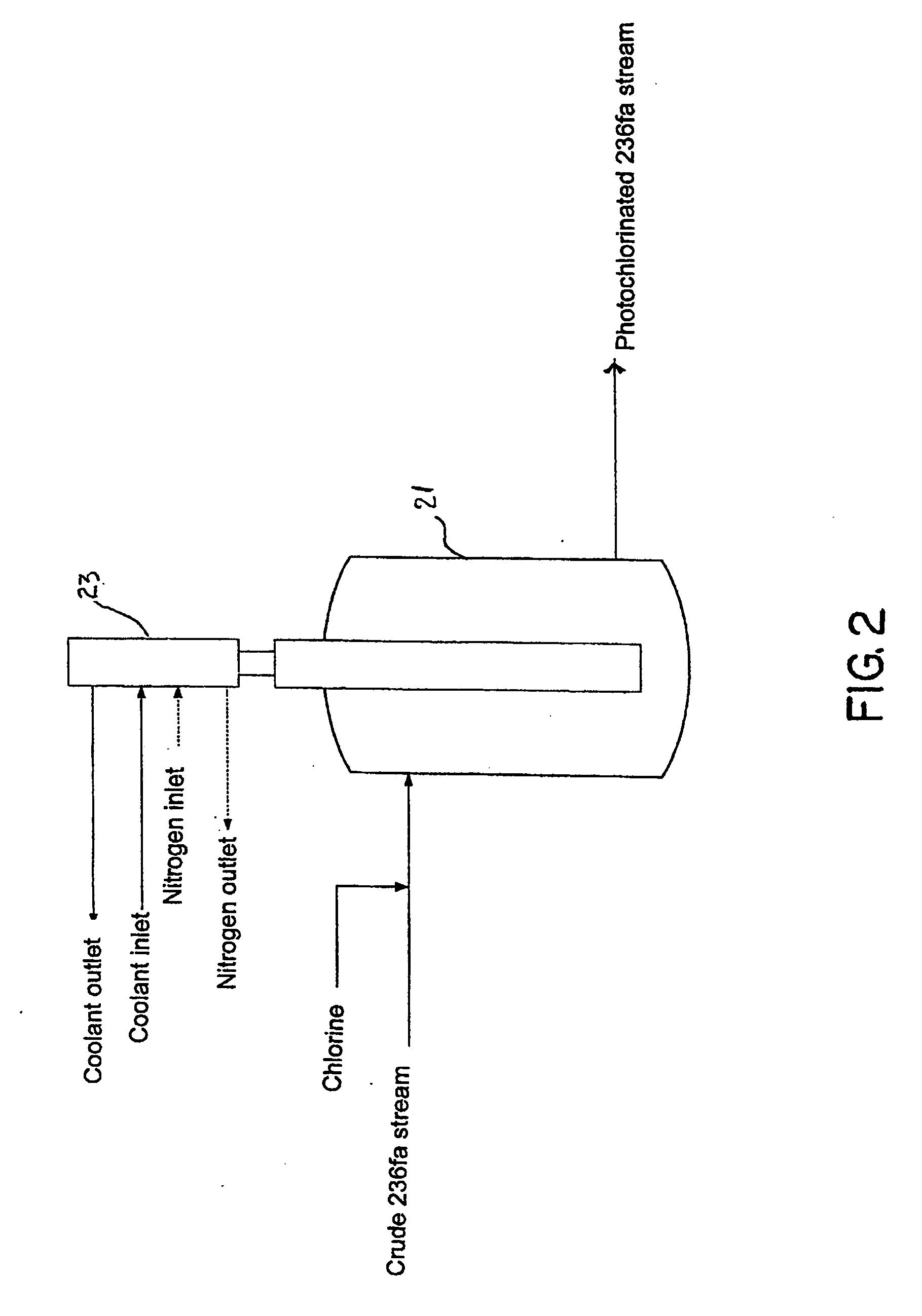
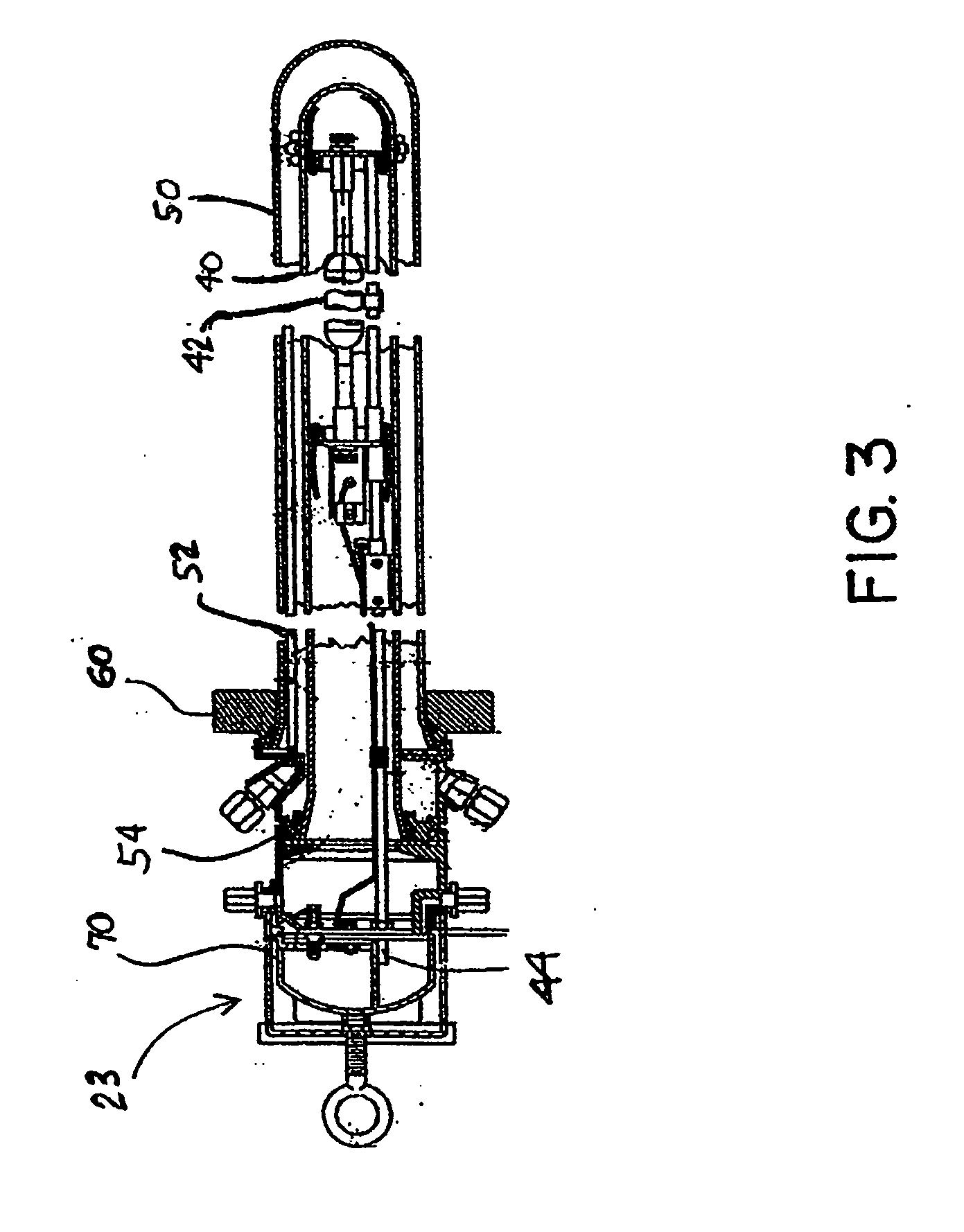
[0028] In accordance with the invention a continuous, vapor phase method is provided to purify HFC-236fa from a crude product mixture containing HFC-236fa and other saturated and unsaturated halocarbons including hydrofluorocarbons (HFC's), chlorofluorocarbons (CFC's) and hydrochlorofluorocarbons (HCFC's), any of which may be saturated or ethylenically unsaturated. The crude product can be produced by several different reaction methods as previously described. The invention comprises a continuous, vapor phase method for purifying a crude mixture of 1,1,1,3,3,3-hexafluoropropane and one or more unsaturated fluorocarbon compounds, the process comprising: [0029] a) providing a photochlorinator vessel comprising [0030] 1) a UV lamp unit comprising a UV lamp located in a transparent inner well, the transparent inner well being located within a transparent outer well, said outer well being provided with material for cooling walls of the inner and outer wells; the inner well and the outer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com