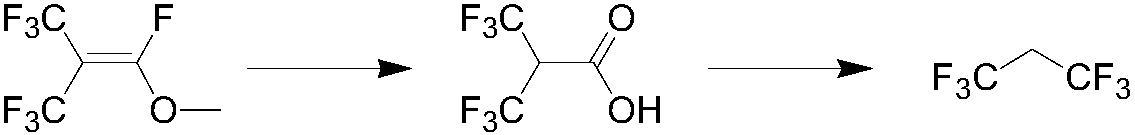
Preparation method of 1, 1, 1, 3, 3, 3-hexafluoropropane
A technology of hexafluoropropane and hexafluoroisobutyric acid, which is applied in the preparation of organic compounds, the preparation of carboxylate, the preparation of halogenated hydrocarbons, etc., can solve the problems of complex preparation process, unfavorable industrialization, and difficulty in obtaining raw materials, etc. The preparation route is short, the industrialization prospect is broad, and the effect of improving the utilization rate of resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1~23
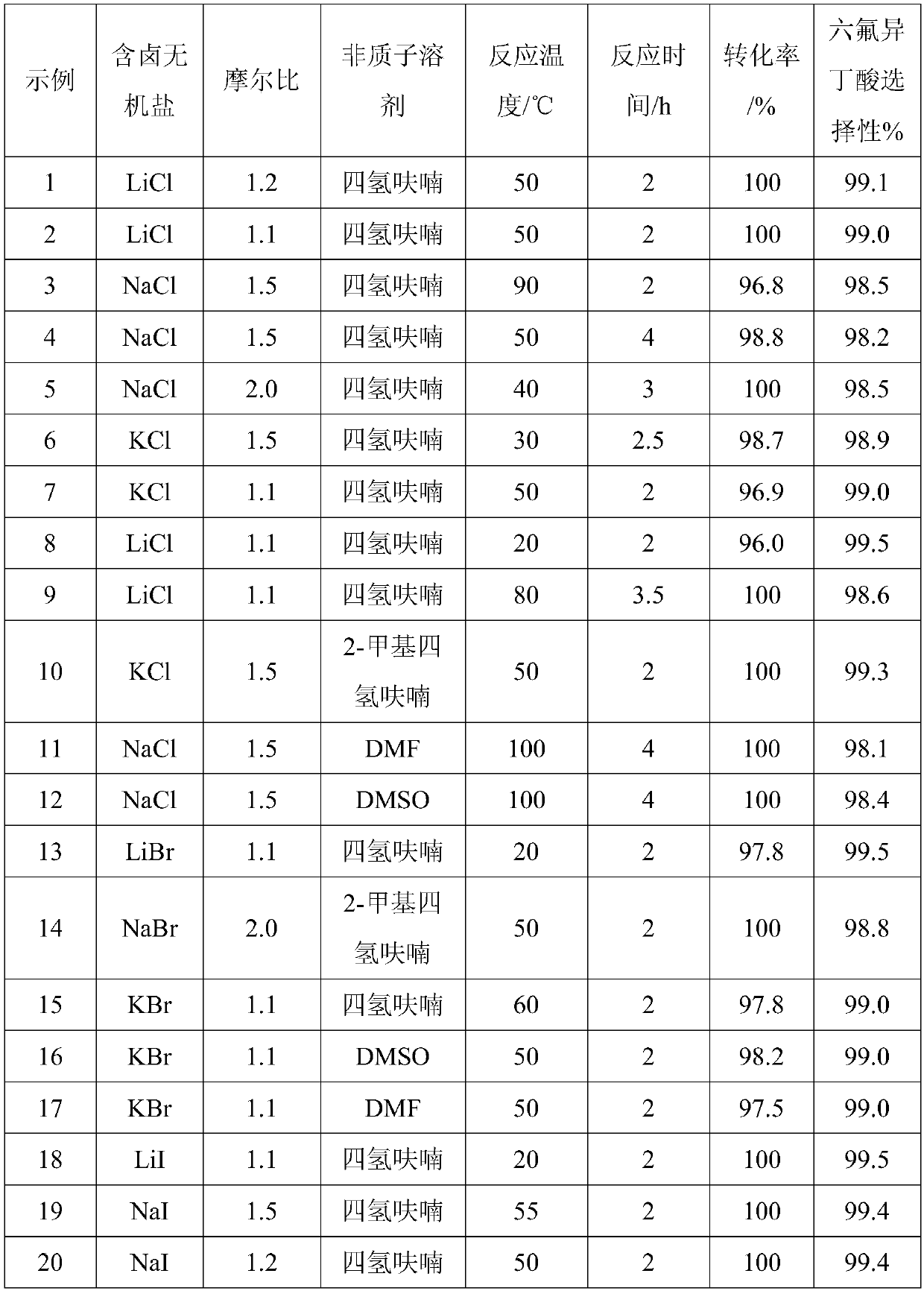
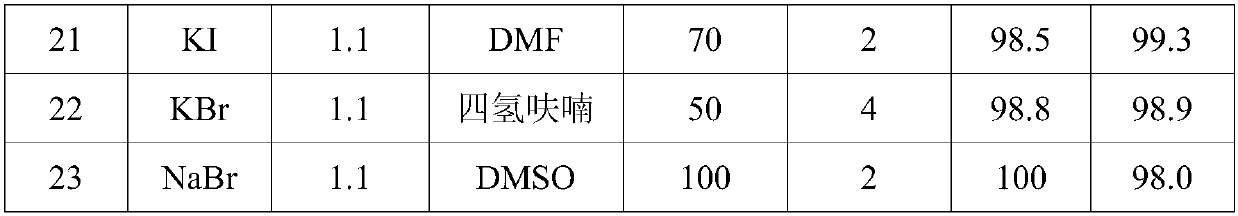
[0037] Under normal pressure stirring conditions, in a 500ml four-necked round-bottomed flask with a condenser, first add a halogen-containing inorganic salt and an aprotic solvent, heat up to the reaction temperature, and then slowly add heptafluoroisobutenyl methyl ether through a constant flow pump to carry out After the reaction, water was added and stirred continuously for 5 minutes, the temperature was lowered and the fluoride salt was filtered off, and the filtrate was rectified to obtain recovered aprotic solvent and hexafluoroisobutyric acid. The test results under different conditions are shown in Table 1.
[0038] Table 1 Hexafluoroisobutyric acid preparation test parameters and results
[0039]
[0040]
[0041] Note: The amount of heptafluoroisobutenyl methyl ether is 106g (0.5mol), the amount of aprotic solvent is 200ml, the amount of water is 0.52mol, and the molar ratio and conversion rate are based on heptafluoroisobutenyl methyl ether.
[0042] Step 2:...
example 24~28
[0044] Take the hexafluoroisobutyric acid prepared in Examples 9-15 and Examples 18-21 respectively, put them in a 500ml three-necked round-bottomed flask with a condenser, heat and stir at the set temperature, and the gas phase products generated are cooled by two stages. , the two-stage cooling temperature is -50°C, and the cooling liquid is collected to obtain the HFC-236fa product. The test results of other different conditions are shown in Table 2.
[0045] Table 2 HFC-236fa preparation test parameters and results
[0046]
[0047] Note: The amount of hexafluoroisobutyric acid is 0.5mol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com