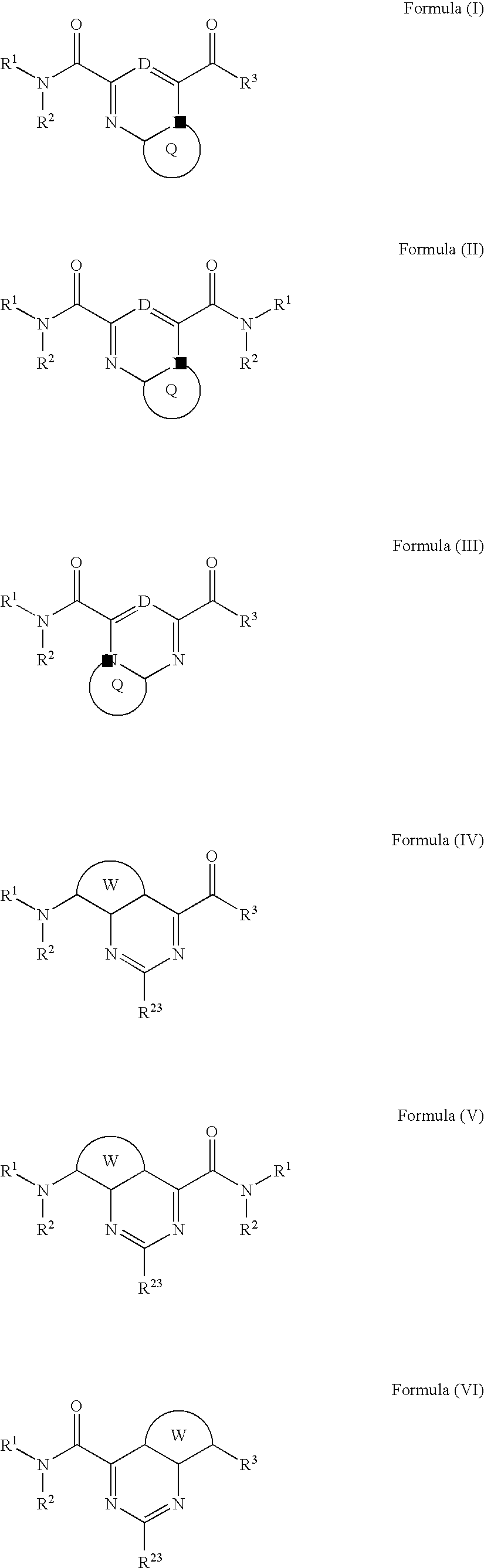
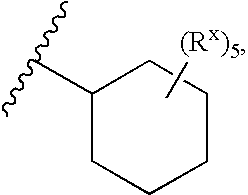
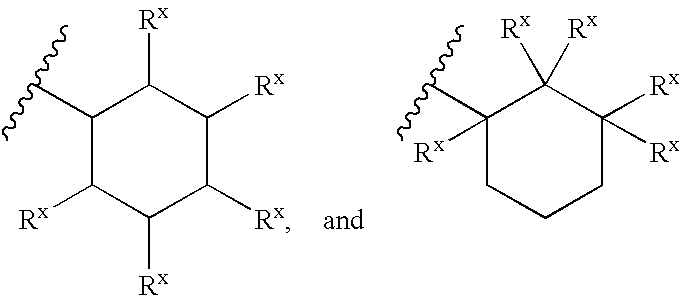
Heterobicyclic metalloprotease inhibitors
a metalloprotease inhibitor and heterobicyclic technology, applied in the field of amide containing heterobicyclic metalloprotease inhibiting compounds, can solve the problems of effective mmp inhibiting compounds, and achieve the effect of treating or preventing metalloprotease, especially
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 8
Preparative Example 8
[0579]
Step A
[0580] To a solution of the title compound from the Preparative Example 3, Step E (153 mg) in EtOH (10 mL) were added NEt3 (0.16 mL) and hydroxylamine hydrochloride (81 mg). The mixture was heated to reflux for 4 h, then concentrated, dissolved in THF (5 mL) and pyridine (0.19 mL) and cooled to 0° C. Trifluoroacetic anhydride (0.25 mL) was added and the mixture was stirred for 16 h. Concentration and purification by chromatography (silica, hexanes / EtOAc) afforded the title compound as a white solid (217 mg, >99%). [MNa]+=392.
example 9
Preparative Example 9
[0581]
Step A
[0582] To a solution of the title compound from the Preparative Example 4, Step A (33.7 mg) in 1,4-dioxane / H2O (1:1, 2 mL) were added NaOH (97.4 mg) and di-tert-butyl dicarbonate (68.7 mg). The resulting mixture was stirred at room temperature overnight, diluted with EtOAc, washed with IN aqueous HCl and saturated aqueous NaCl, dried (MgSO4), and concentrated to give a white solid (34.6 mg, 71%). [MNa]+=300.
Step B
[0583] To a solution of the title compound from Step A above (34.6 mg) in CH2Cl2 (1 mL) were added oxalyl chloride (33 μL) and DMF (2 μL). The mixture was stirred at room temperature for 2 h and concentrated. The remaining residue was dissolved in CH2Cl2 (1 mL) and added to a cold (−78° C.) saturated solution of NH3 in CH2Cl2 (1 mL). The mixture was stirred at −78° C. for 1 h, warmed to room temperature, concentrated, redissolved in CH2Cl2 (5 mL), filtered, and concentrated to give a white solid (25.9 mg, 75%). [MNa]+=299.
example 10
Preparative Example 10
[0584]
Step A
[0585] To mixture of the title compound from the Preparative Example 7, Step B (536 mg) and allyl bromide (1.6 mL) in CHCl3 / THF (1:1, 20 mL) were added Bu4NHSO4 (70 mg) and a 1M solution of LiOH in H2O (10 mL) and the resulting biphasic mixture was stirred at 40° C. overnight. The organic phase was separated, concentrated, diluted with CHCl3, washed with H2O, dried (MgSO4), filtered, concentrated and purified by chromatography (silica, cyclohexane / EtOAc) to afford the title compound (610 mg, >99%). [MNa]+=354.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Structure | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
| Stereoisomer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com