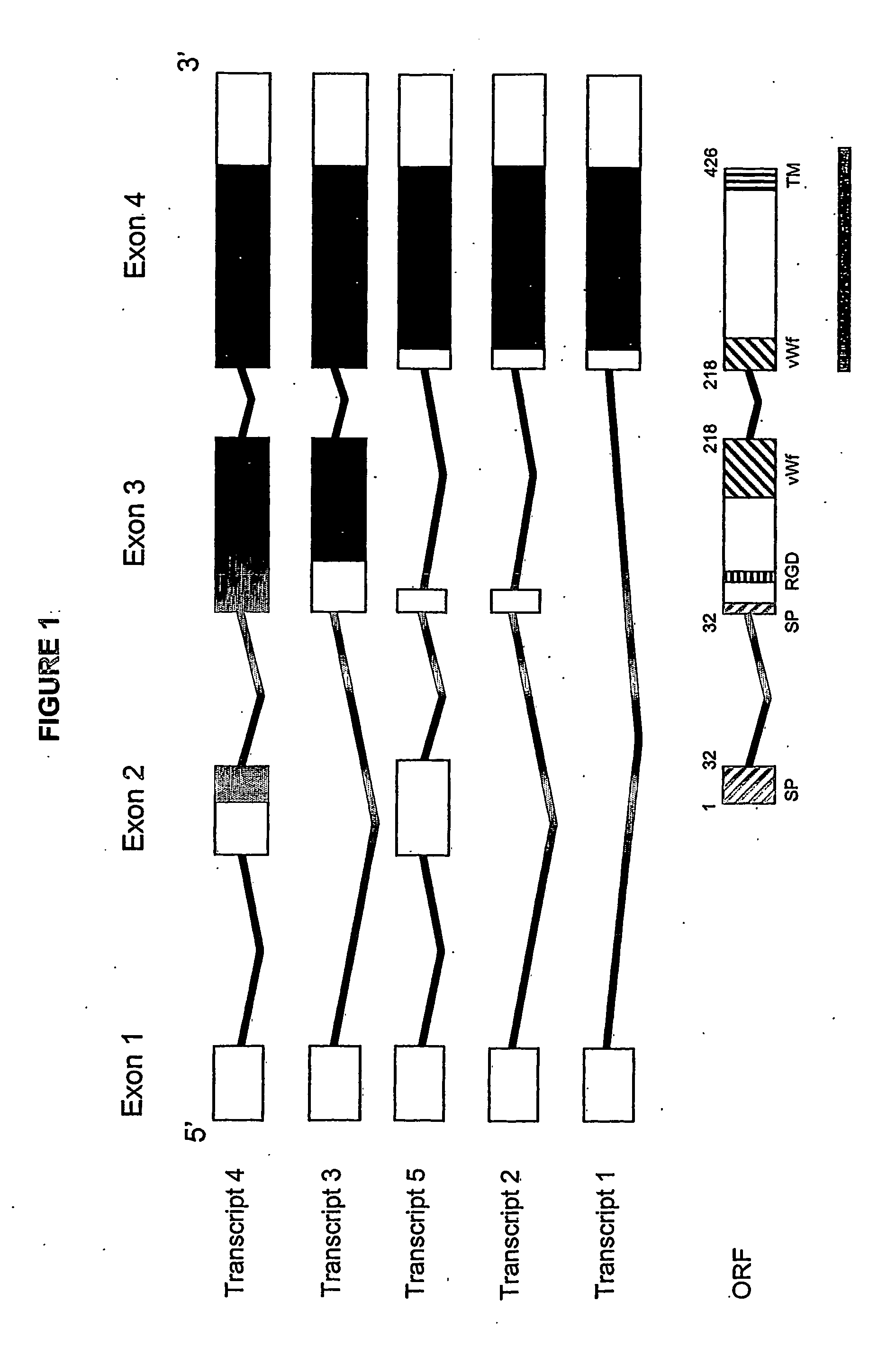
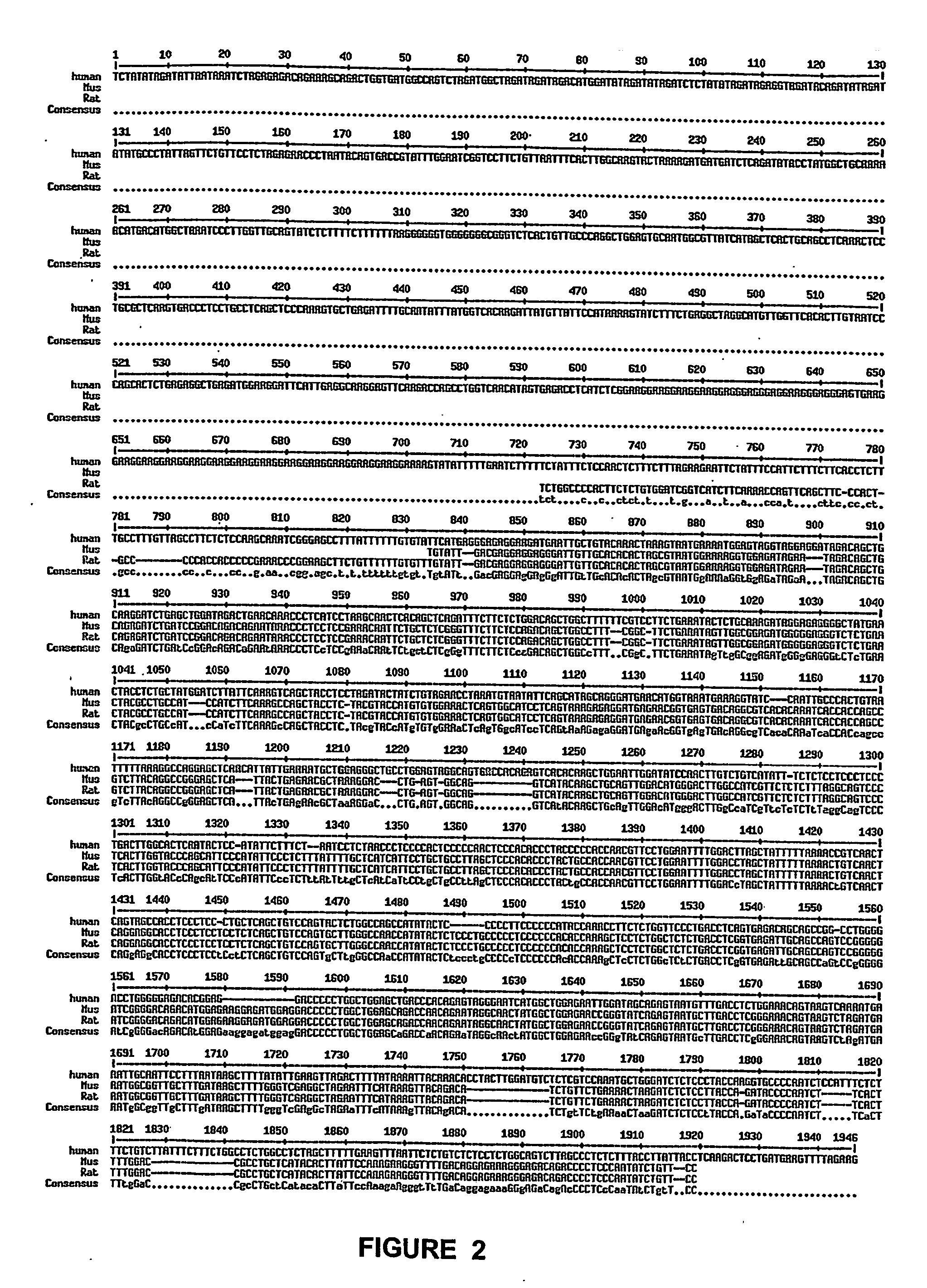
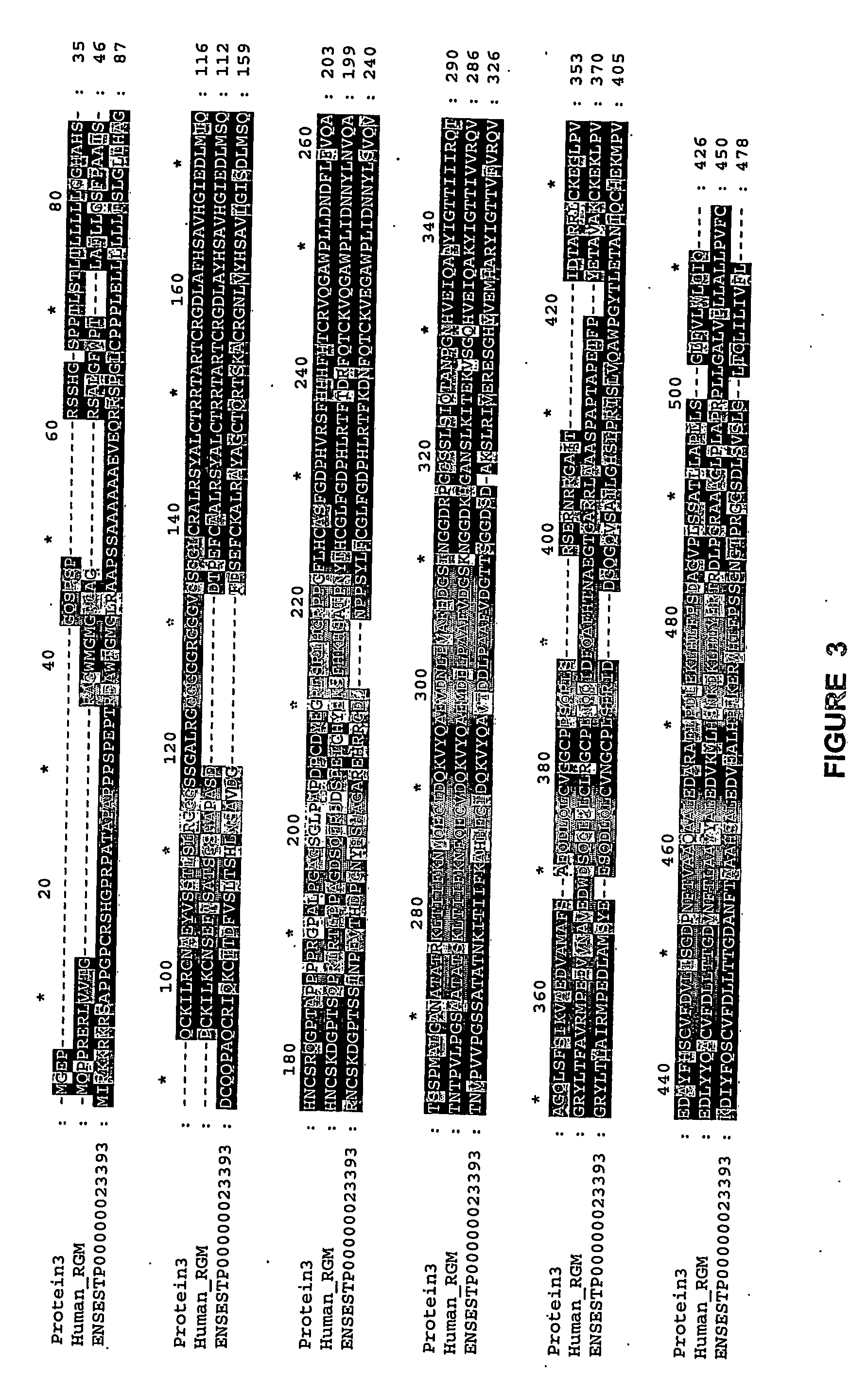
Juvenile hemochromatosis gene (hfe2a), expression products and uses thereof
a technology of juvenile hemochromatosis and gene, applied in the field of juvenile hemochromatosis, can solve the problem of lethal disease, and achieve the effect of high degree of similarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of the Genetic Mutation Responsible for Juvenile Hemochromatosis 2A (HFE2A).
[0232] 3. We have collected ten Greek families including 13 individuals with JHH. Pedigrees of these families are shown in FIGS. 9a-9d. Of these families (JH3-12), five have been reported previously (JH3-7) (Papanikolaou, G. et al. Linkage to chromosome lq in Greek families with juvenile hemochromatosis. Blood Cells Mol. Dis. 27, 744-749 (2001)). Only one family, JH7, is known to be consanguineous. We confirmed that the disease is consistent with linkage to 1 q21 (HFE2A; OMIM 602390) in the new families by using 25 genotype markers, including six new microsatellite markers identified from genomic sequence. While the Build 31 human genome sequence assembly contains gaps and duplications, we were able to estimate the size of the linkage interval, and define the linkage boundaries and gene content based on existing sequence contigs. Homozygosity mapping in JH7 defined the limits of the candidate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com