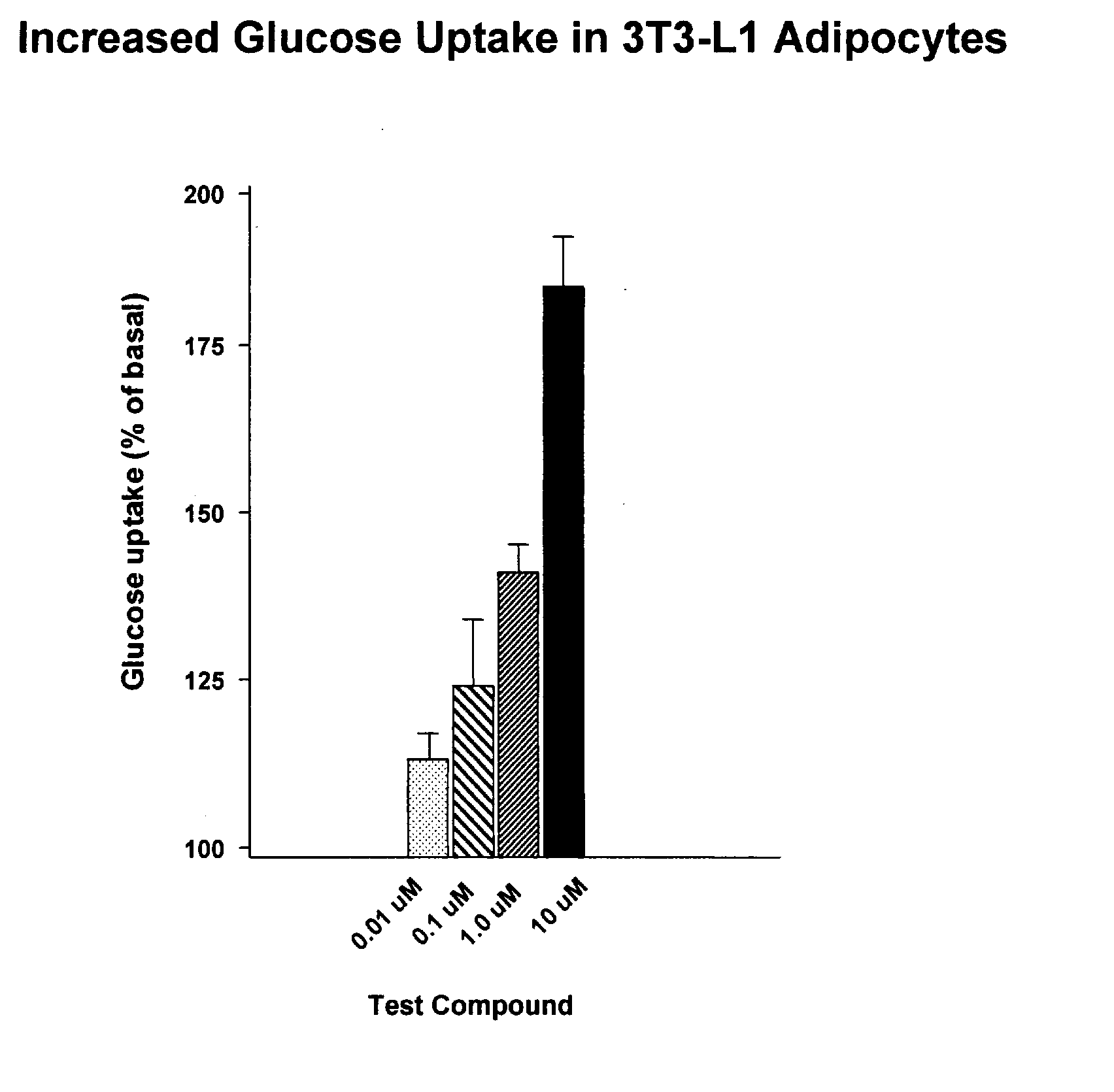
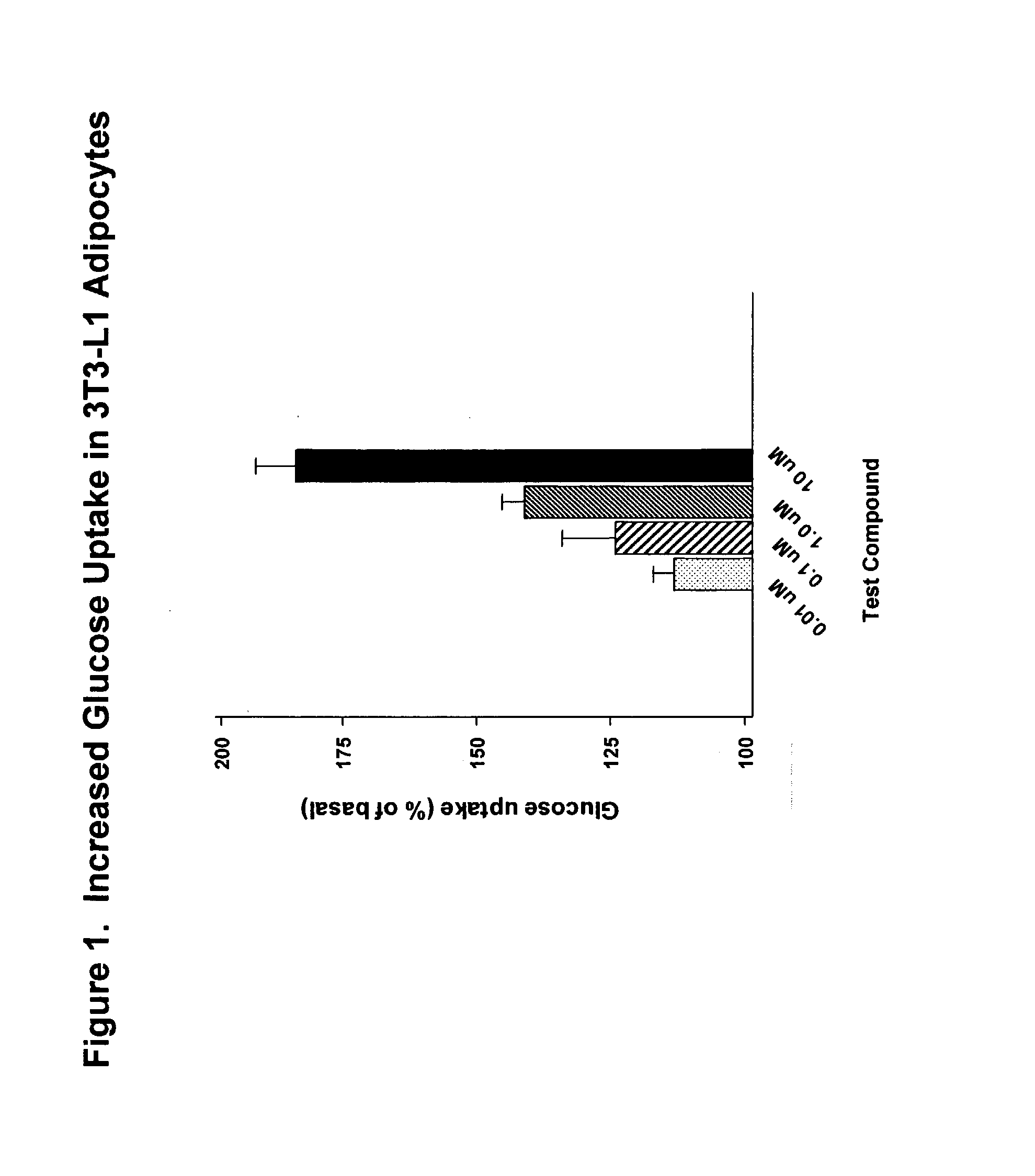
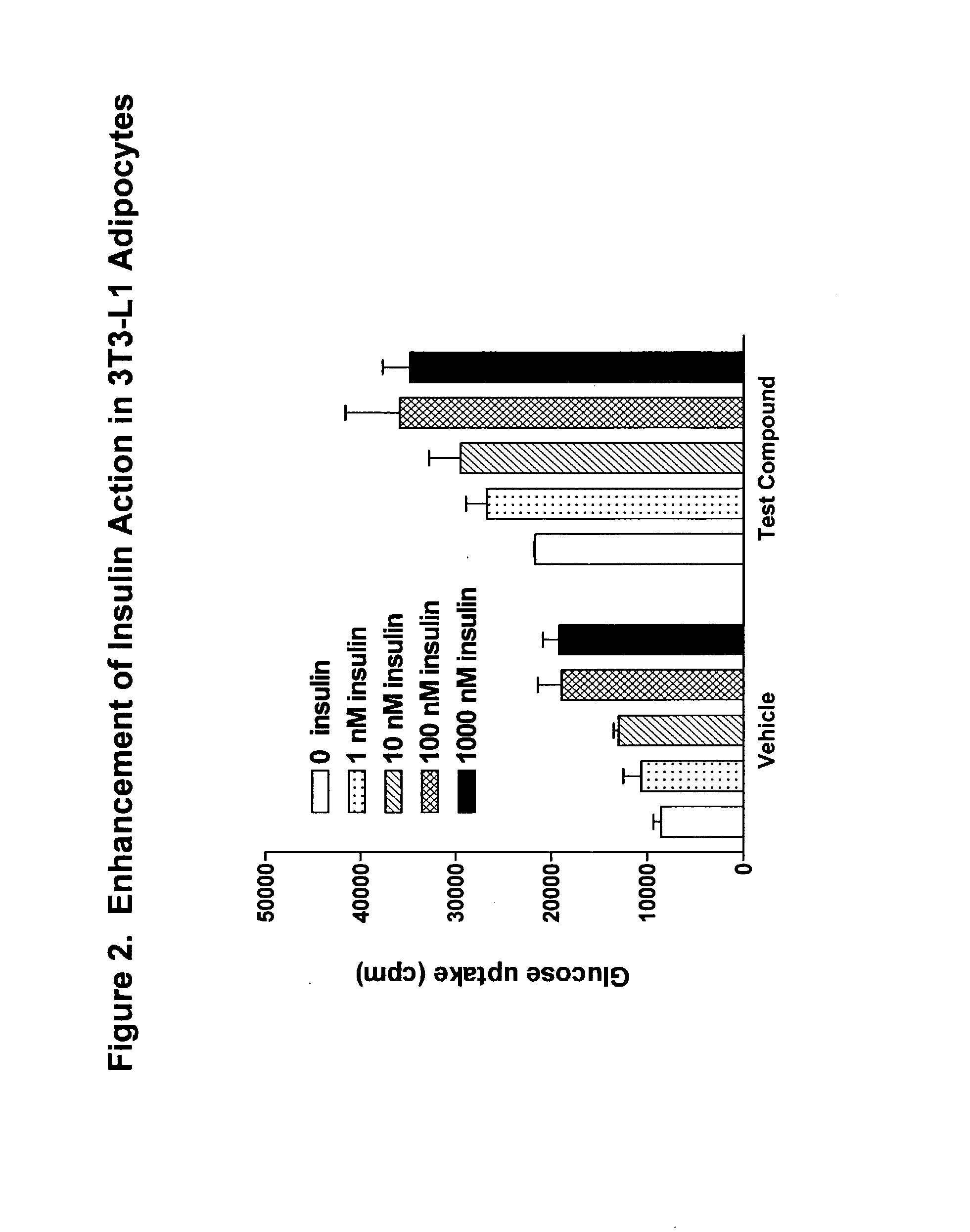
Compounds for treatment of inflammation, diabetes and related disorders
a technology for inflammation and diabetes, applied in the field of compounds for the treatment of inflammation, diabetes and related disorders, can solve the problems of morbidity and mortality, undesirable side effects of gastrointestinal pathology, and the ineffectiveness of known thiazolidinediones for a significant portion of the patient population
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 3-(3,5-Dimethoxyphenyl)-2-{4-[4-(3-Oxo-3-Ureidopropyl)-Phenoxy]-Phenyl}-Acrylic Acid Methyl Ester (1) [see Scheme I]
[0165] Step 1: Synthesis of 3-(3 5-dimethoxyphenyl)-2-(4-hydroxyphenyl)-acrylic acid (2). To a mixture of 3,5-dimethoxybenzaldehyde (120 g, 0.72 mol) and p-hydroxyphenyl acetic acid (110 g, 0.72 mol) was added acetic anhydride (240 mL) and triethylamine (161 mL, 1.6 equiv.). This non-homogeneous mixture on heating becomes homogeneous at ˜70° C. After being stirred at 130° C. for 4 hr, the mixture was cooled to room temperature. HCl (15%, 500 mL) was added to the reaction mixture slowly in 30min keeping temperature below 5-10° C. The solid was dissolved in 3N aqueous NaOH (1.2 L) and stirred for 0.5 hr. The filtrate was acidified, maintaining a temperature at 25-30° C., with conc. HCl (˜700 mL) to pH 1. The precipitated product was filtered and washed with water to give crude product (˜300 g, wet cake). The crude product was dissolved by heating in ethanol...
example 2
Synthesis of 3-(3,5-Dimethoxyphenyl)-2-{4-[4-(3-Ethoxycarbonylamino-3-Oxo-Propyl)-Phenoxy]-Phenyl}-Acrylic Acid Methyl Ester (8)
[0171] 2-{4-[4-(2-Carbamoyl-ethyl)-phenoxy]-phenyl }-3-(3,5-dimethoxyphenyl)-acrylic acid methyl ester (7) was obtained as a byproduct in the synthesis of 3-(3,5-dimethoxy-phenyl)-2-{4-[4-(2,4-dioxothiazolidin-5-ylmethyl)-phenoxy]-phenyl}-acrylic acid methyl ester, performed essentially as shown in PCTIUS99 / 09982 (WO 99 / 58127). 7 (460 mg, 1.0 mmol) was taken up in dry THF (6 mL) and cooled to −78° C. To this solution, lithium diisopropyl amide (LDA) (2M, 0.55 mL, 1.1 mmol) was added and stirred for 10 min. Ethyl chloroformate (0.11 mL, 1.2 mmol) was added and stirred overnight at room temperature. The reaction was quenched with saturated aqueous ammonium chloride solution and ethyl acetate (50 mL) was added. The organic layer was washed with brine (2×20 mL), dried on anhydrous magnesium sulfate and evaporated under reduced pressure. The crude product was ...
example 3
Synthesis of 2-{4-[4-(3-Benzoyloxycarbonylamino-3-Oxo-Propyl)-Phenoxy]-Phenyl}-3-(3,5-Dimethoxyphenyl)-Acrylic Acid Methyl Ester (9)
[0173] 7 (1.38, 3.0 mmol) prepared as in Example 2 was taken up in dry THF (20 mL) and cooled to −78° C. To this solution, LDA (2M, 1.8 mL, 3.6 mmol) was added and stirred for 10 min. Benzyl chloroformate (0.67 g, 39 mmol) was added and stirred overnight at room temperature. The reaction was quenched with saturated aqueous ammonium chloride solution, and ethyl acetate (150 mL) was added. The organic layer was washed with brine (2×25 mL), dried on anhydrous magnesium sulfate and evaporated under reduced pressure. The crude product was purified by silica gel chromatography and eluted with hexane-ethyl acetate (7:3). Yield: 0.68g, 37.3%.
[0174] Analysis: 1HNMR (DMSO-d6): δ 10.65 (s, 1H), 7.72 (s, 1H), 7.38-7.39 (m, 5H), 7.25 (d, J=8.4 Hz, 2H), 7.18 (d, J=8.4 Hz, 2H), 7.00 (d, J=8.4 Hz, 2H), 6.94 (d, J=8.4 Hz, 2H), 6.41 (t, J=2.0 Hz, 1H), 6.28 (d, J=2.0 ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap