N,N-dialkyl-S-hydroxyalkyl-dithiocarbamate collecting agent and preparation method and application thereof
A technology of dithiocarbamate and hydroxyalkyl is used in N,N-dialkyl-S-hydroxyalkyl-dithiocarbamate collectors and the fields of preparation and application thereof, achieving Good reaction effect, improved dispersion and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
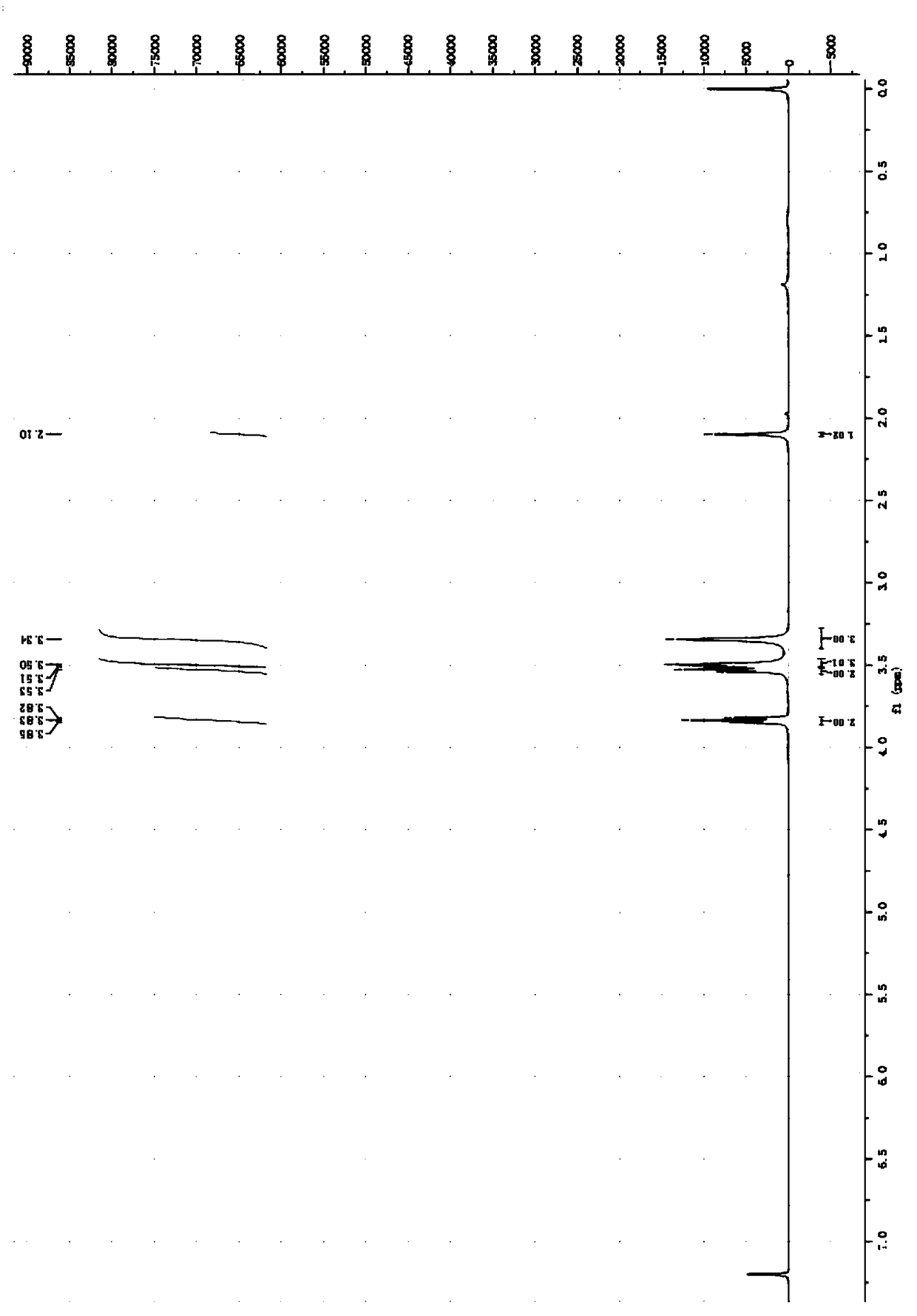
[0056] Embodiment 1: the preparation of N, N-dimethyl-S-hydroxyethyl-dithiocarbamate
[0057] Add 30.65 parts of carbon disulfide with a purity of 99% into a three-necked flask, add 4.38 parts of sodium hydroxide with a purity of 96% in batches under ice bath, stir mechanically, then add 11.27 parts of an aqueous solution of dimethylamine with a purity of 40%, dropwise The time is 40 minutes, the dropping process controls the temperature not to exceed 10°C, and then the temperature is raised to 15°C in the bottle, and after constant temperature reaction for 1 hour, 8.13 parts of 2-chloroethanol with a purity of 99% are added, and the temperature in the bottle is raised to 50°C. After constant temperature reaction for 5 hours, it was cooled to room temperature. Wash 3 times with 100mL deionized water, then extract with 100mL dichloromethane, separate the liquid to obtain an oily phase liquid, then treat it with anhydrous sodium sulfate overnight, spin evaporate and separate and...
Embodiment 2
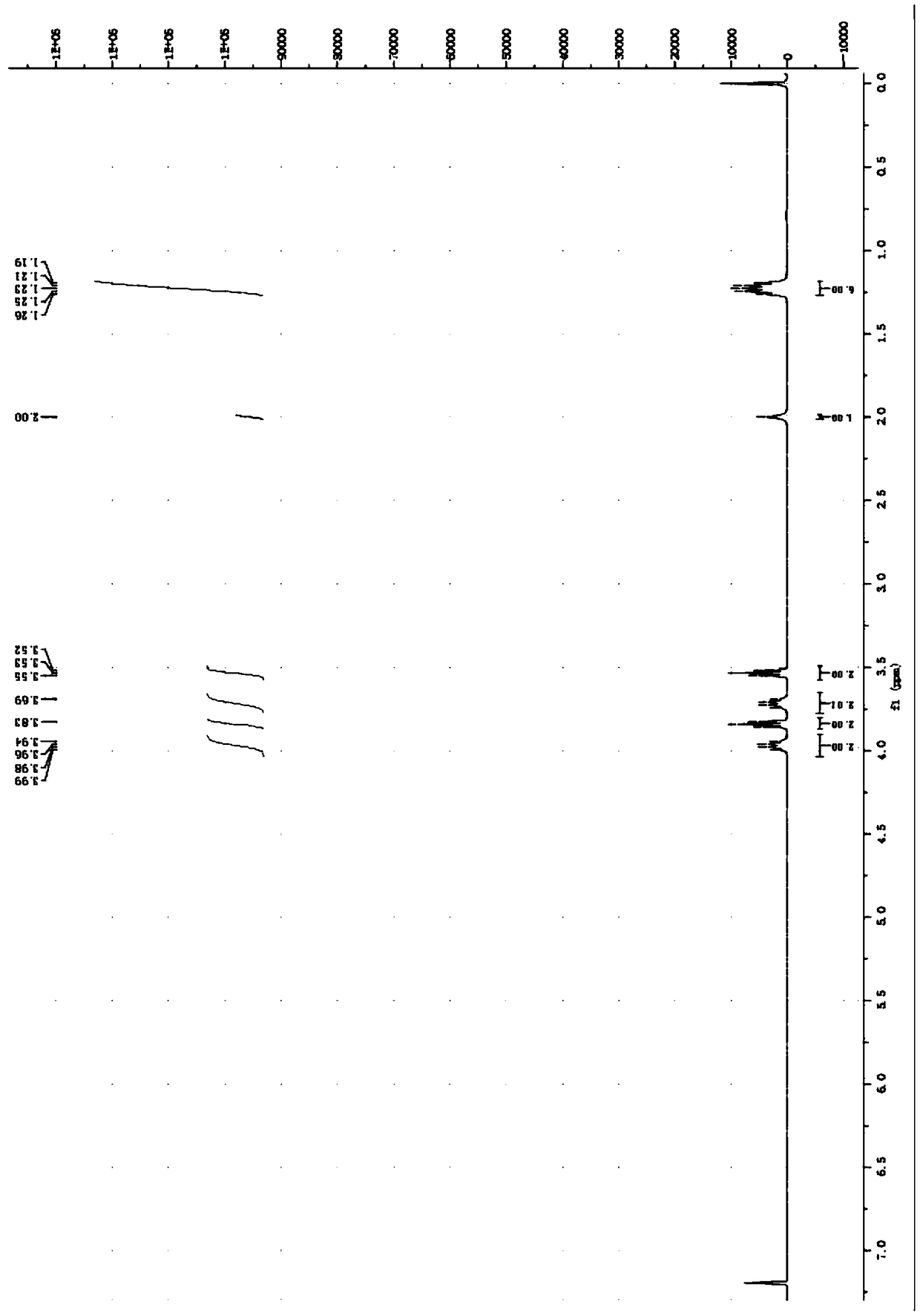
[0058] Embodiment 2: the preparation of N, N-diethyl-S-hydroxyethyl-dithiocarbamate
[0059] Add 30.65 parts of carbon disulfide with a purity of 99% into a three-necked flask, add 4.38 parts of sodium hydroxide with a purity of 96% in batches under an ice bath, stir mechanically, then add 5.4 parts of deionized water dropwise, and then add 7.39 parts of 99% pure water dropwise. % of diethylamine, the dropping time is 40 minutes, the dropping process controls the temperature not to exceed 10°C, then raises the temperature to 15°C in the bottle, and adds 8.13 parts of 2-chloroethanol with a purity of 99% after 1 hour of constant temperature reaction, and heats up When the temperature in the bottle was 50°C, after 5 hours of constant temperature reaction, it was cooled to room temperature. Wash 3 times with 100mL deionized water, then extract with 100mL dichloromethane, separate the liquid to obtain an oil phase liquid, then treat it with anhydrous sodium sulfate overnight, and ...
Embodiment 3
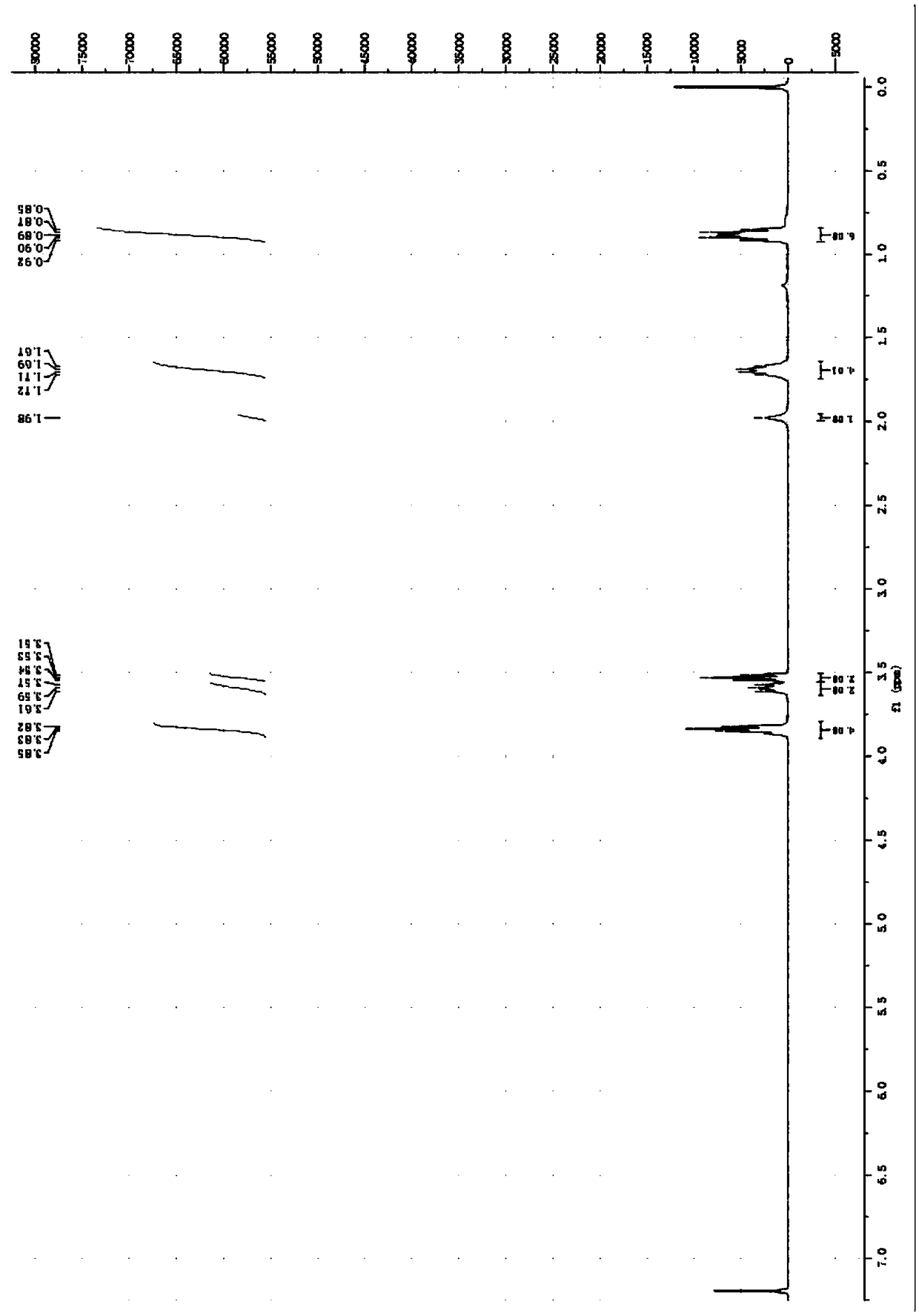
[0060] Embodiment 3: Preparation of N, N-di-n-propyl-S-hydroxyethyl-dithiocarbamate
[0061] Add 30.65 parts of carbon disulfide with a purity of 99% into a three-necked flask, add 4.38 parts of sodium hydroxide with a purity of 96% in batches under an ice bath, stir mechanically, then dropwise add 5.4 parts of deionized water, and then add dropwise 10.22 parts of a purity of 99% % of di-n-propylamine, the dropping time is 40 minutes, the dropping process controls the temperature not to exceed 10°C, and then raises the temperature to 15°C in the bottle, and after constant temperature reaction for 1 hour, add 8.13 parts with a constant pressure dropping funnel with a purity of 99% 2-chloroethanol, control the reaction temperature to be 50°C, cool to room temperature after reacting for 5 hours, separate the liquid to obtain the oil phase N, N-di-n-propyl-S-hydroxyethyl-dithiocarbamate, use Wash with 100 mL of deionized water for 3 times, then extract with 100 mL of dichlorometha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com