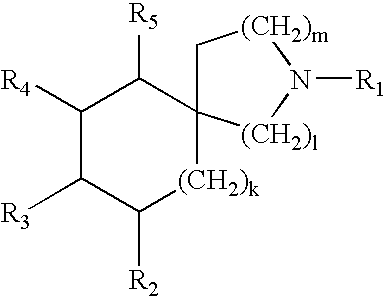
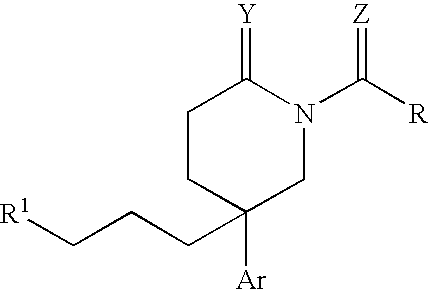
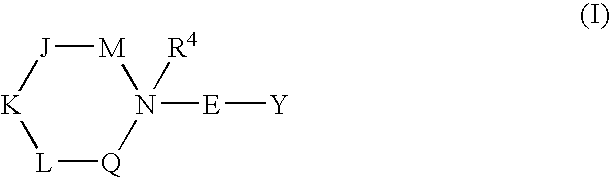Heterocyclic piperidines as modulators of chemokine receptor activity
a technology heterocyclic piperidines, which is applied in the field of modulators of chemokine receptor activity, can solve the problems of not being disclosed nor suggested in the prior ar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of (+ / −)-N-phenyl-2-[[4-[(4-fluorophenyl)methyl]-1-piperidinyl]methyl]-1-piperidinecarboxamide
Step A. Preparation of N-(t-butoxycarbonyl)-4-(4-fluorophenylmethalene)piperidine.
[0587] To a stirring solution of 4-fluorobenzyl-triphenylphosphonium chloride (61.3 g, 150.6 mmol, Fluka) in dry THF (500 mL) was added a 1M solution of potassium t-butoxide (138 mL). The reaction was stirred for 5 min and then a solution of N-(t-butoxycarbonyl)-4-piperidone (25 g, 125.5 mmol) in dry THF (100 mL) was added. After 10 min, the reaction was warmed to reflux for 16 h. After cooling to room temperature, the reaction was conc. in vacuo to an oil. The oil was dissolved in EtOAc and hexanes was added to form a white precipatate. The precipatate was filtered off and the filtrate conc. in vacuo to an oil. The oil was purified by flash chromatography (SiO2, 0-35% EtOAc in hexanes) to yield 27 grams (74%) of the product as a white solid. MS (ESI) 314 (M+Na).
Step B. Preparation of N-(t-but...
example 2
Preparation of (+ / −)-N-(3-cyanophenyl)-2-[[4-[(4-fluorophenyl)methyl]-1-piperidinyl]methyl]-1-piperidinecarboxamide
[0595] Prepared according to procedures described in Example 1 with modification at Step H. MS (ESI) 435 (M+H).
example 3
Preparation of (+ / −)-N-(3-methoxyphenyl)-2-[[4-[(4-fluorophenyl)methyl]-1-piperidinyl]methyl]-1-piperidinecarboxamide
[0596] Prepared according to procedures described in Example 1 with modification at Step H. MS (ESI) 440 (M+H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com