Packaging and method for selective volume dispensing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
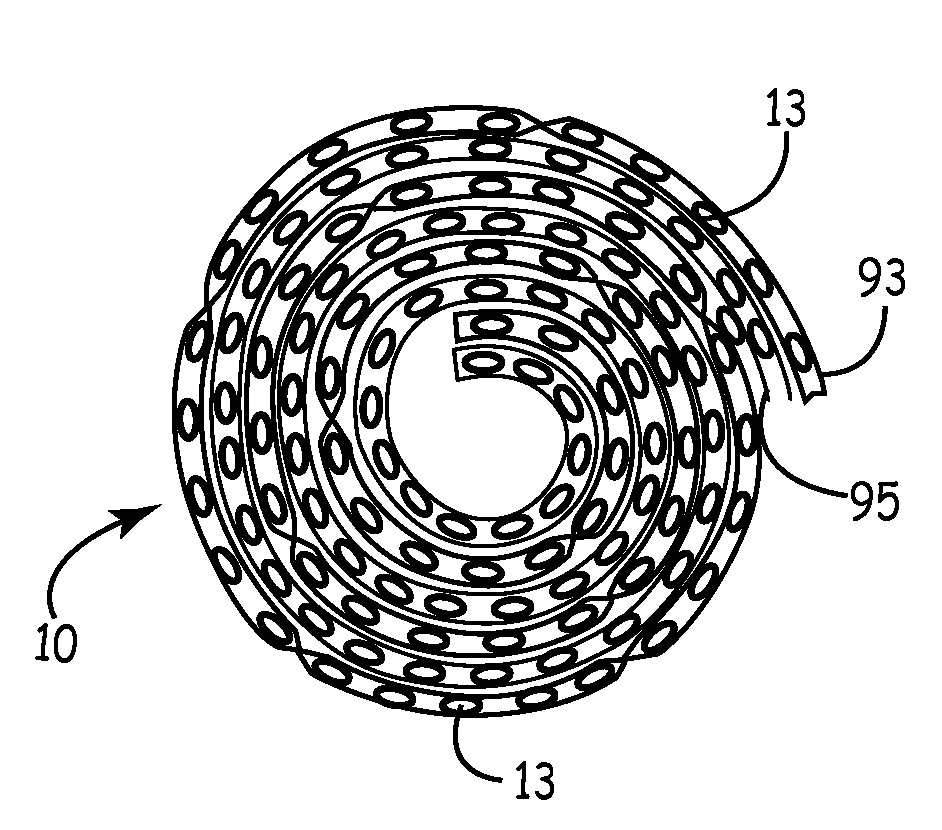
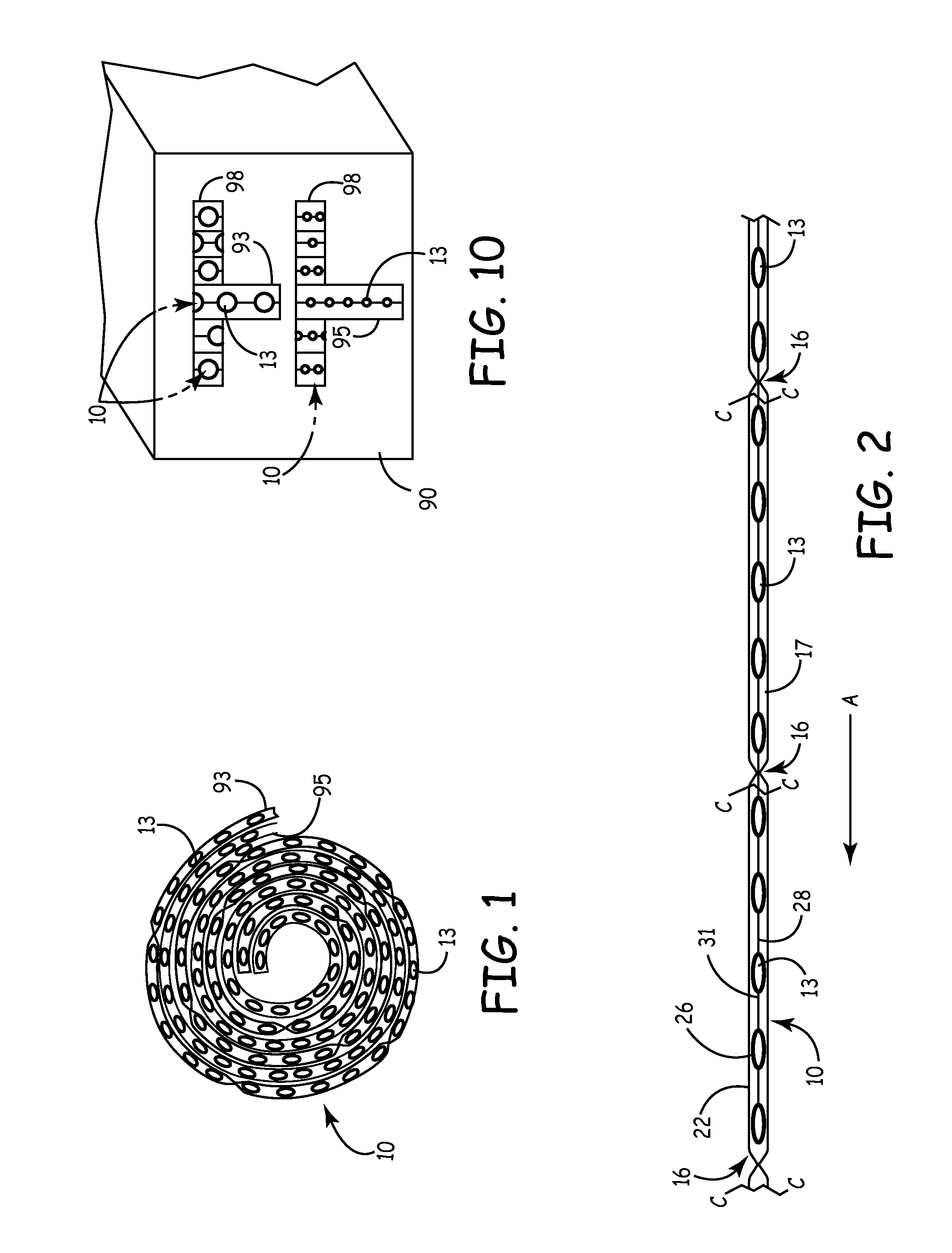
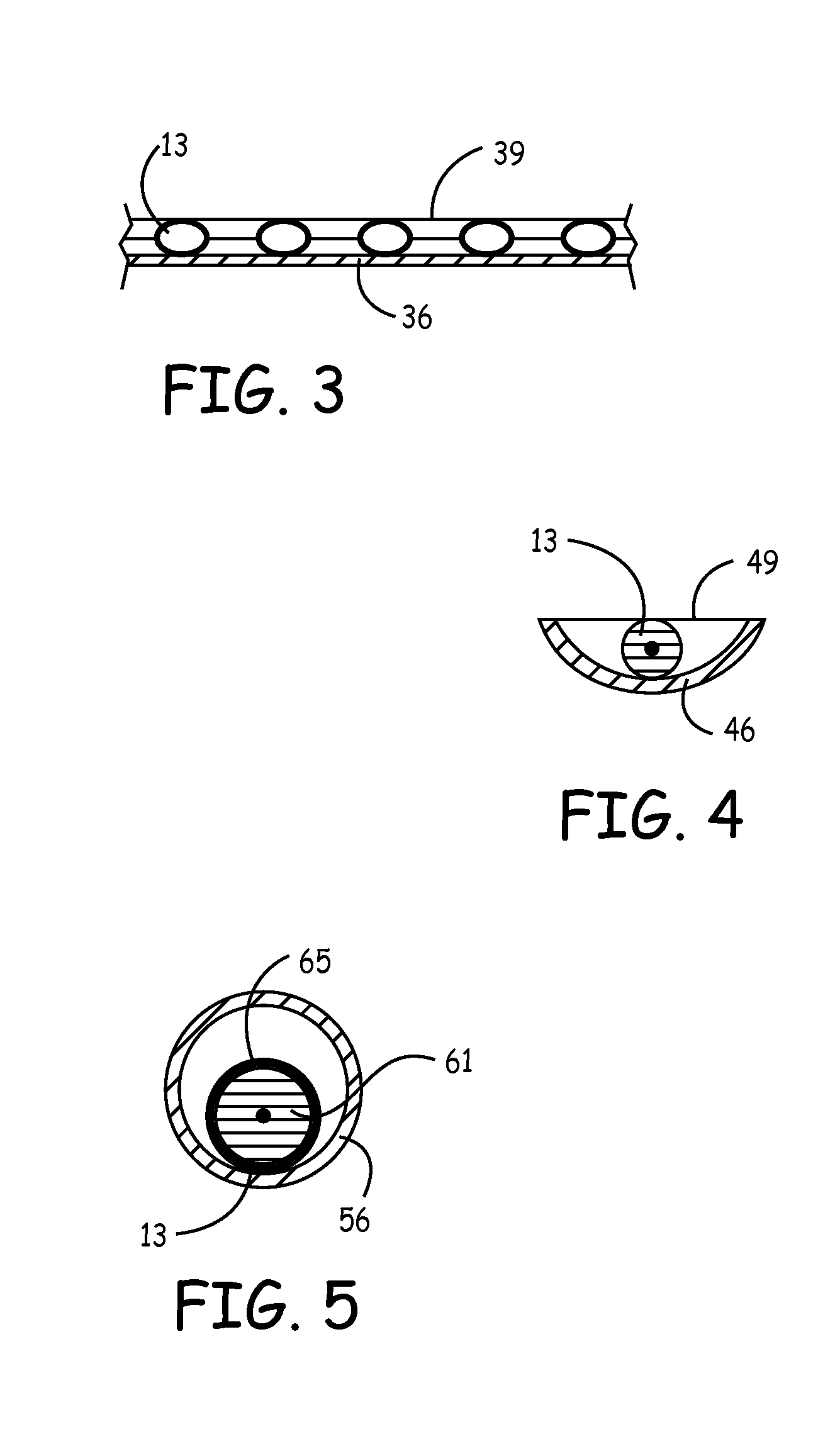
[0022]It is well known that sterile primary packaging of medical devices, soft goods, human consumption products or pharmaceuticals is preferably a single or unitary package having a set amount (often just one) of devices therein. However, this requirement may be unnecessarily wasteful or inefficient. Such inefficiencies increase the cost of healthcare and reduce productivity and profitability in this field. In one example, such excessive costs are tolerated in view of the paramount importance of maintaining a safe, sterile, and known quality product. In a second example, such costs create disincentives and impediments to using the best products and devices. Packaging improvements are needed to facilitate efficiencies and to accommodate unique product requirements.
[0023]Within the field of medical devices and other products for either ingestion or critical exposure to mammals, such devices and products must be handled with a heightened standard of care. In particular, some products ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com