Hydrogen-oxygen gas generator and hydrogen-oxygen gas generating method thereof
a hydrogen-oxygen gas and generator technology, applied in the field of apparatus and methods for generating hydrogen-oxygen gas, can solve the problems of limited efficiency of the apparatus and method of the related art, inability to provide adequate efficiency, and limited amount of hydrogen-oxygen gas produced by one hydrogen-oxygen gas generator, etc., to achieve satisfactory uniformity, high efficiency, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
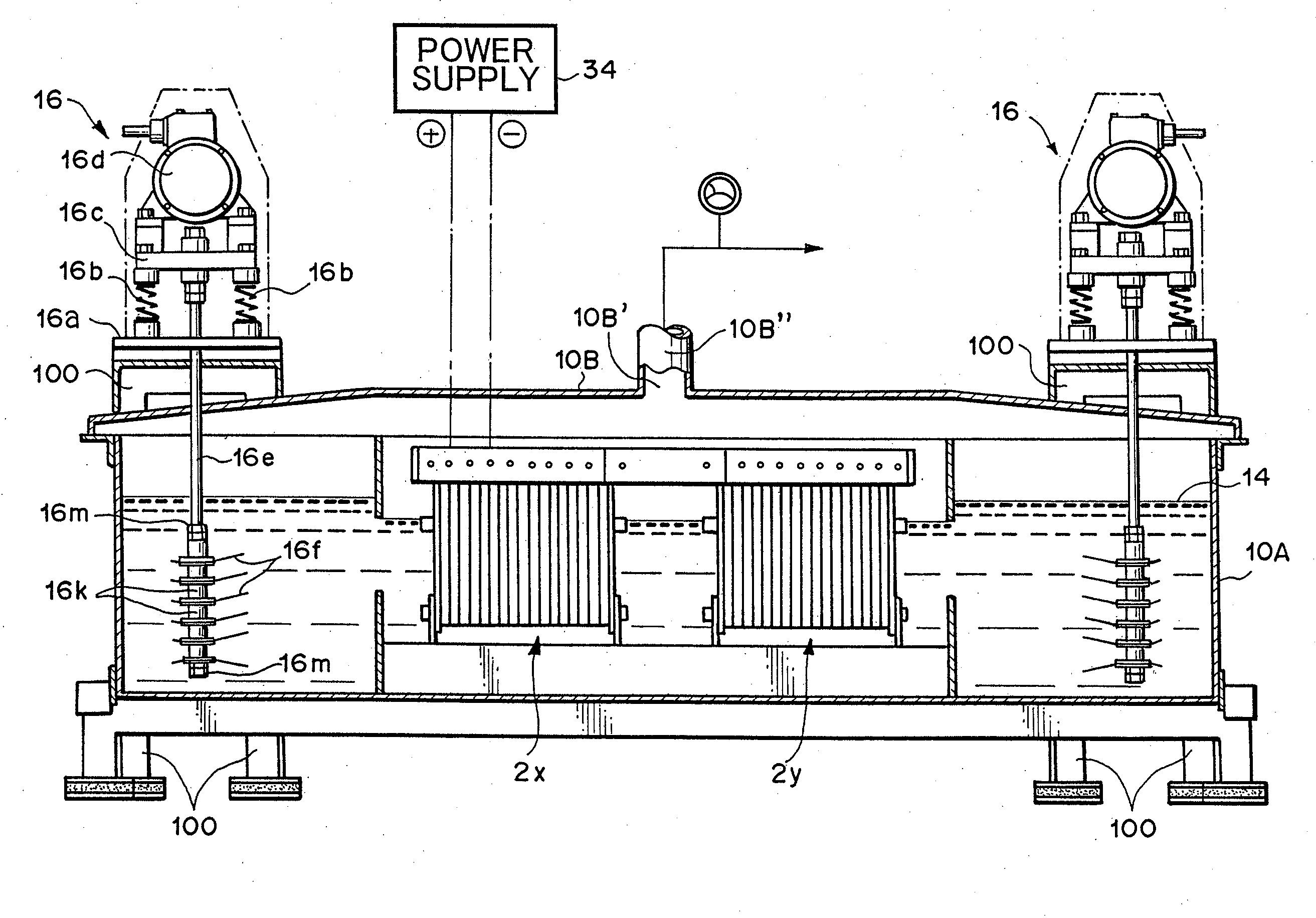
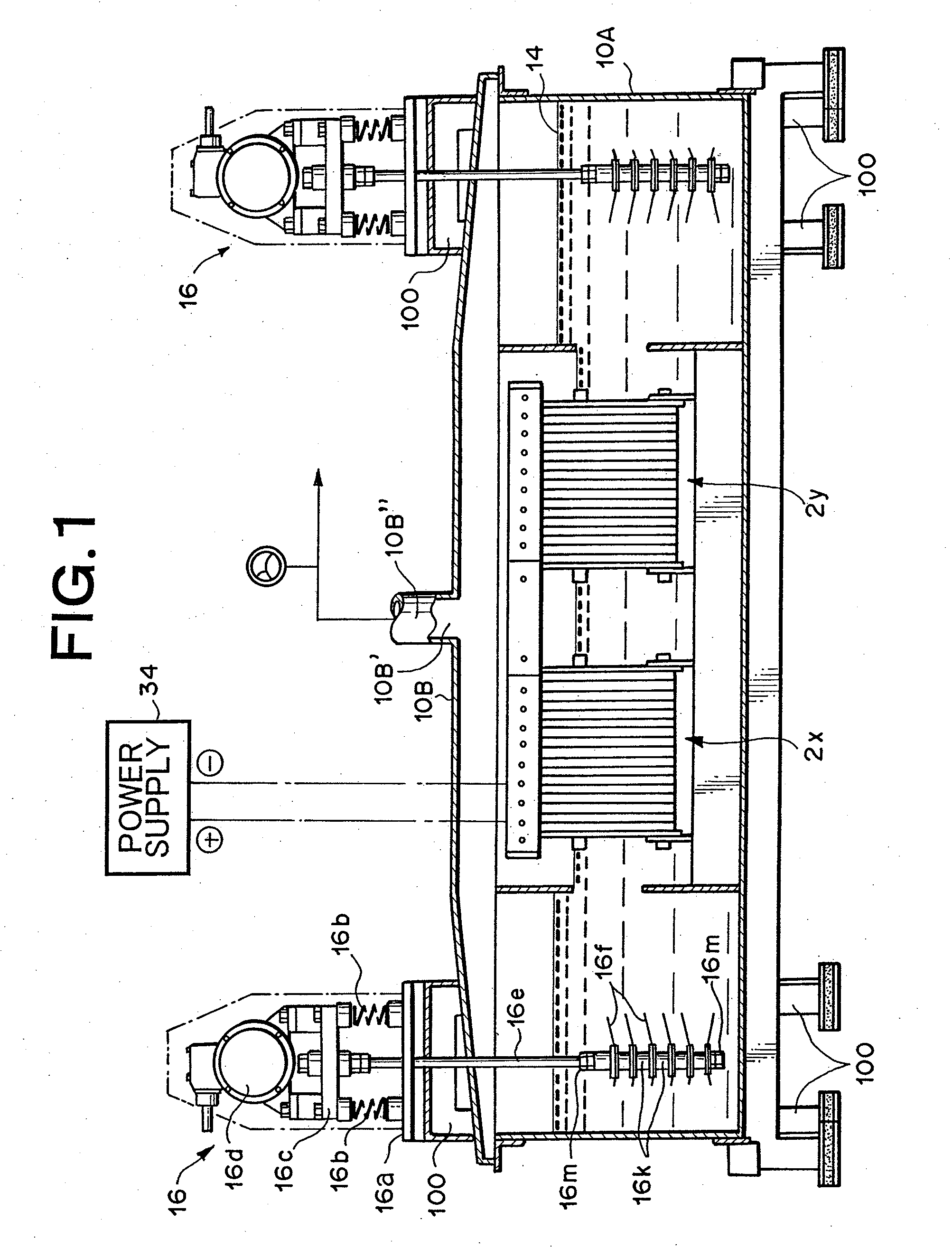
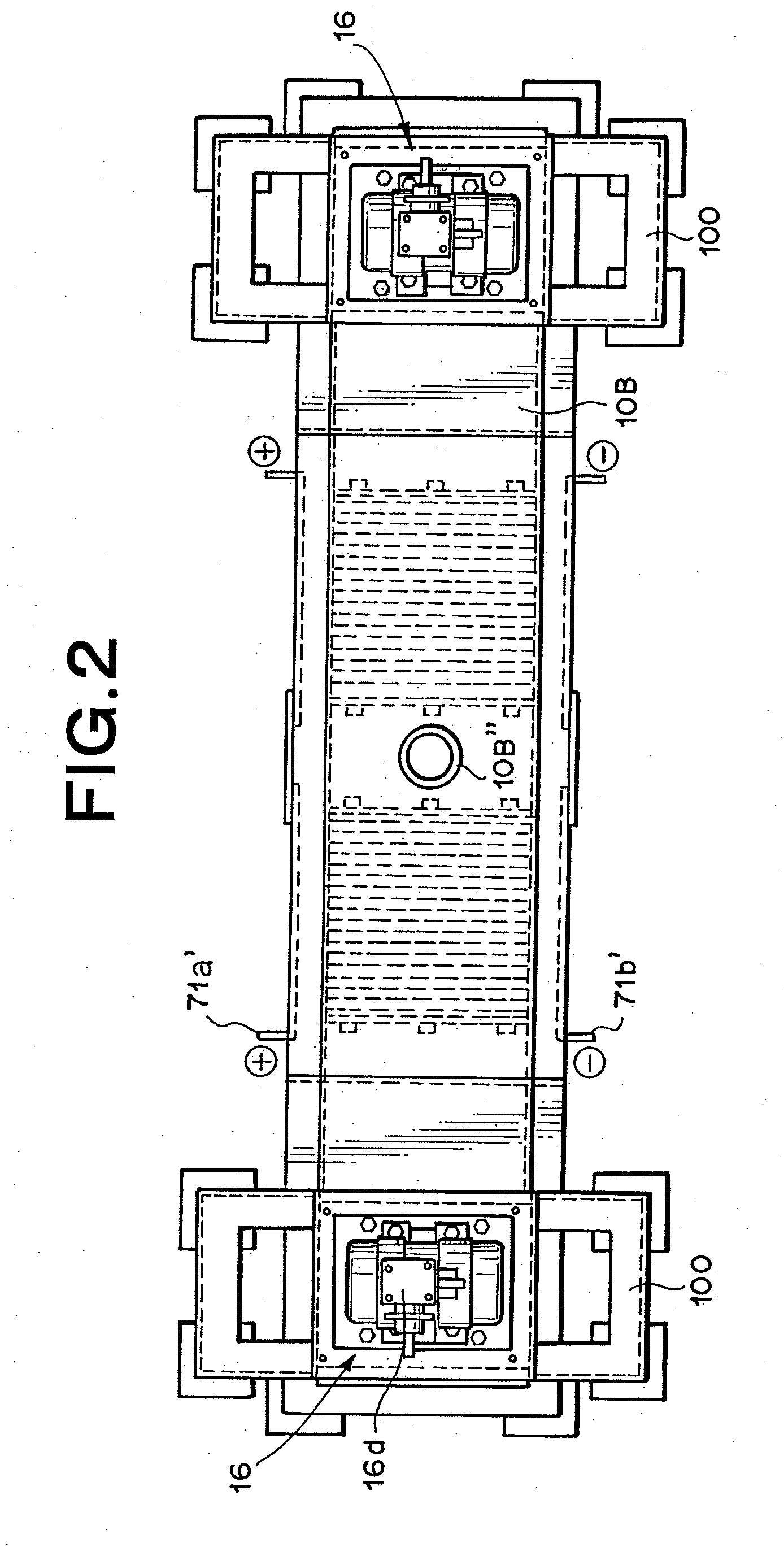
[0097]Utilizing the device as described in FIG. 1 through FIG. 3, but with the lid member 10B described in FIG. 29, hydrogen-oxygen gas was generated and collected under the following conditions.
Electrolytic Cell and Lid Member:
[0098]Manufactured from stainless steel
[0099]270 mm×1660 mm×390 mm (H)
Vibration Generating Means:
[0100]Vibration motor; Uras Vibrator manufactured by Murakami Seild Seisakusho (Corp.) (product name), 250 W×3-phase×200 V, 2-axis type,
[0101]Vibrating blades: Manufactured from stainless steel (SUS304), 6 blades
[0102]Vibrating rod: Manufactured from titanium, 12 mm diameter
[0103]Spacers: 12 pieces, manufactured from titanium
[0104]Clamp members for vibrating blades; 12 pieces
[0105]Packing for vibrating blades: 12 sheets, manufactured by Teflon (registered trademark)
Electrode Group:
[0106]Anodes: 50 sheets, made from platinum plated titanium alloy capable of long-term use without film oxidation
[0107]Cathodes: 50 sheets, made from titanium alloy
[0108]Insulation frame...
second embodiment
[0113]Other than utilizing an AC multiplex current as described in “Electrochemistry” (Society of Japan) Vol. 24, P. 398-403, and pages 449-456 of same volume, the same structure as in the first embodiment was utilized.
[0114]Hydrogen-oxygen gas collection rate was 1,200 liters per hour.
[0115]After continuous operation over a period of one month, stable collection of hydrogen-oxygen gas was achieved at a power consumption lower than the first embodiment.
third embodiment
[0116]Other than using a 270 mm×850 mm×340 mm (H) structure as the electrolytic cell, and using one Hifrerrous KHE-2-2T [100 to 120 Hz] unit manufactured by Murakami Seiki Seisakusho (Corp.) (product name) as the vibration motor, the same structure as in the first embodiment was utilized.
[0117]Hydrogen-oxygen gas collection rate was 800 liters per hour.
PUM
| Property | Measurement | Unit |
|---|---|---|
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| vibration frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com