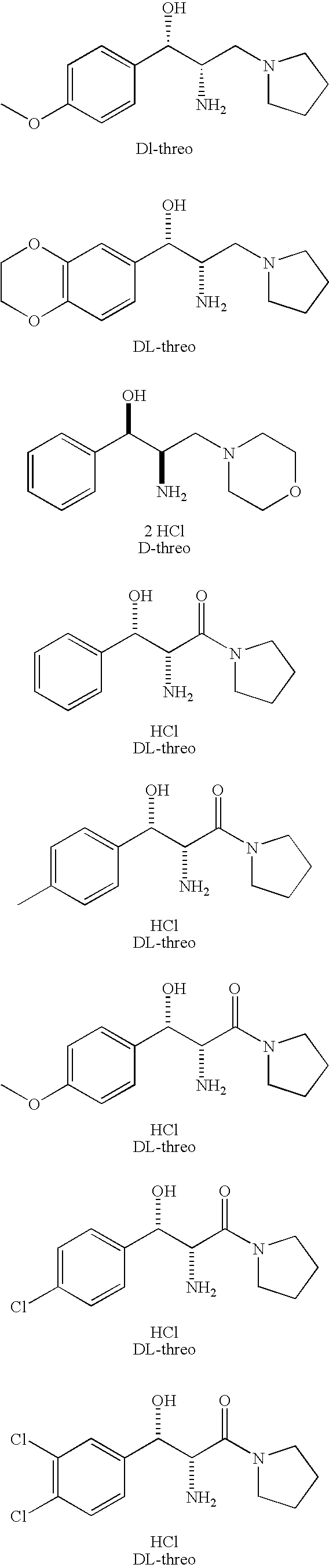
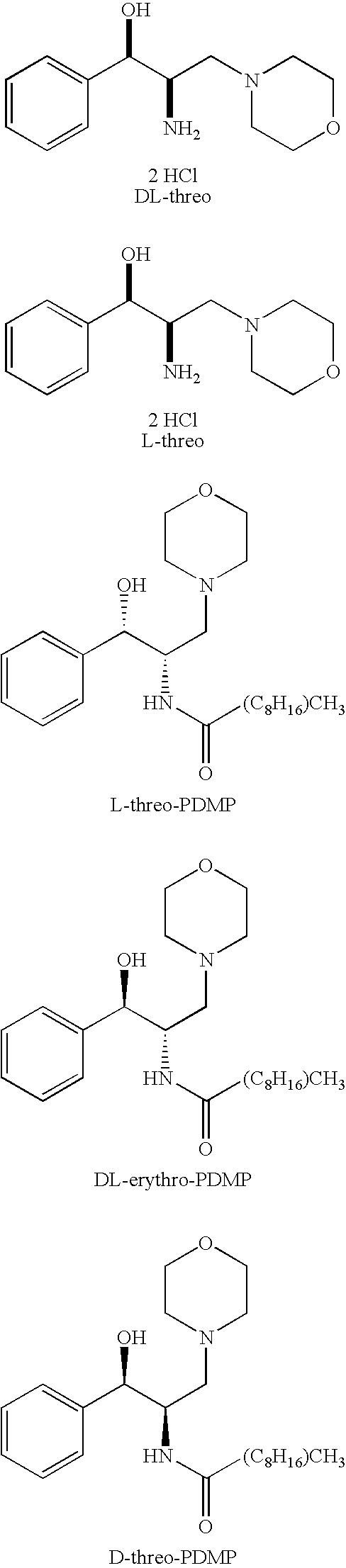
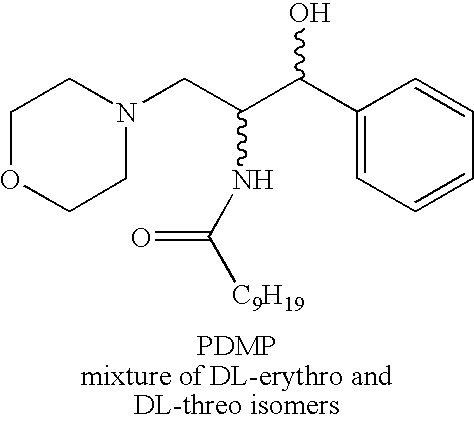
Methods of using as analgesics 1-benzyl-1-hydroxy-2,3-diamino-propyl amines, 3-benzyl-3-hydroxy-2-amino-propionic acid amides and related compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019]The chemical structures of the compounds used in the methods and compositions of the present invention are provided in the Summary Section of the present application for patent. All of compounds used in the methods and compositions of the present invention have two asymmetric carbons adjacent to one another and therefore, generally speaking, can exist in erythro or threo form, with each of these two forms having dextrorotatory (D) or levorotary (L) enantiomers. Nevertheless, most compounds presently used in the methods and compositions of the present invention are in the threo form which itself can have dextrorotatory (D) or levorotary (L) enantiomers. The scope of the present invention includes use of the threo and erythro isomers, mixtures of erythro and threo isomers, both enantiomers of the isomers in optically pure form, racemic mixtures and mixtures where the enantiomers are not present in equal amounts. In light of the foregoing, it should be clearly understood that the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com