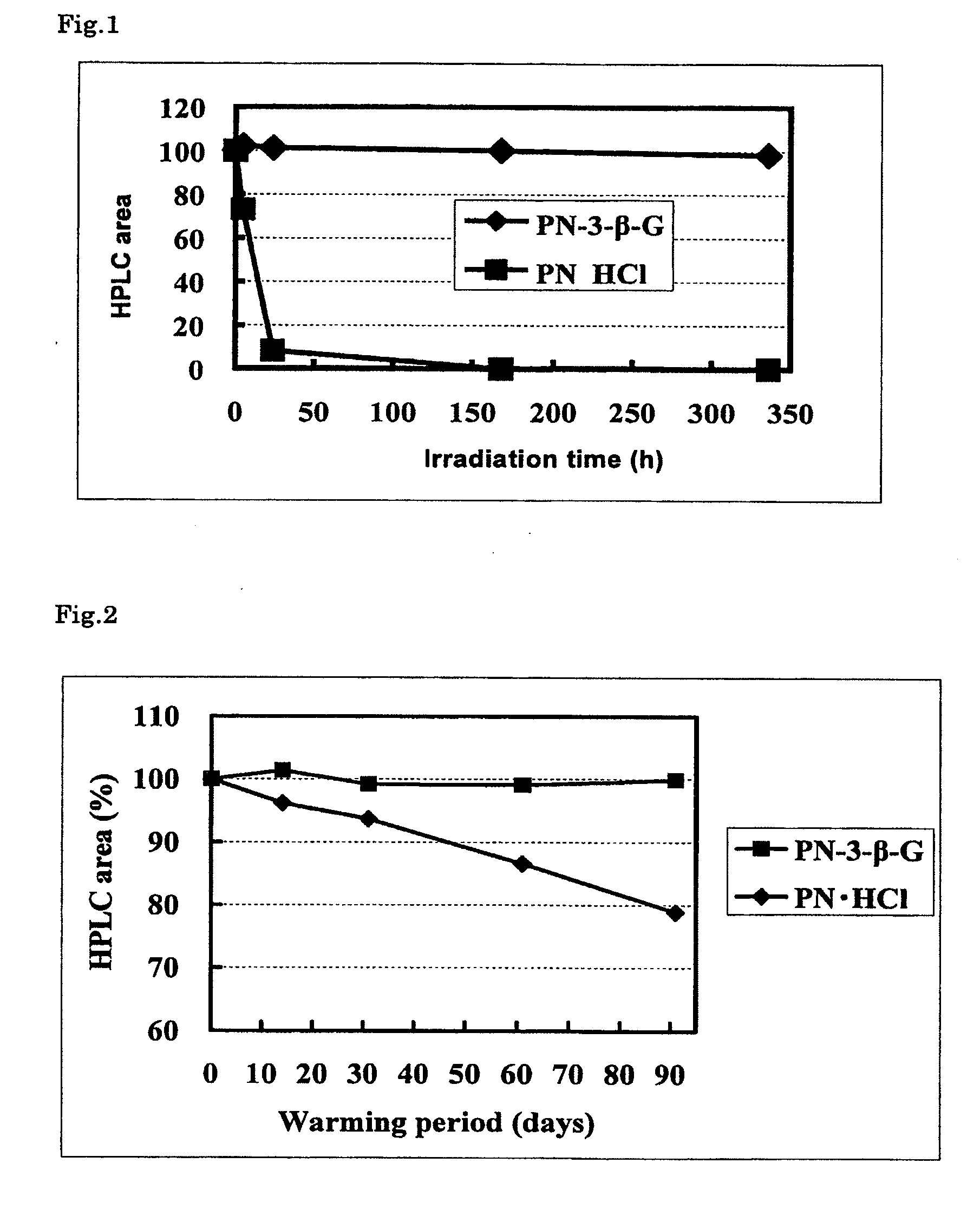
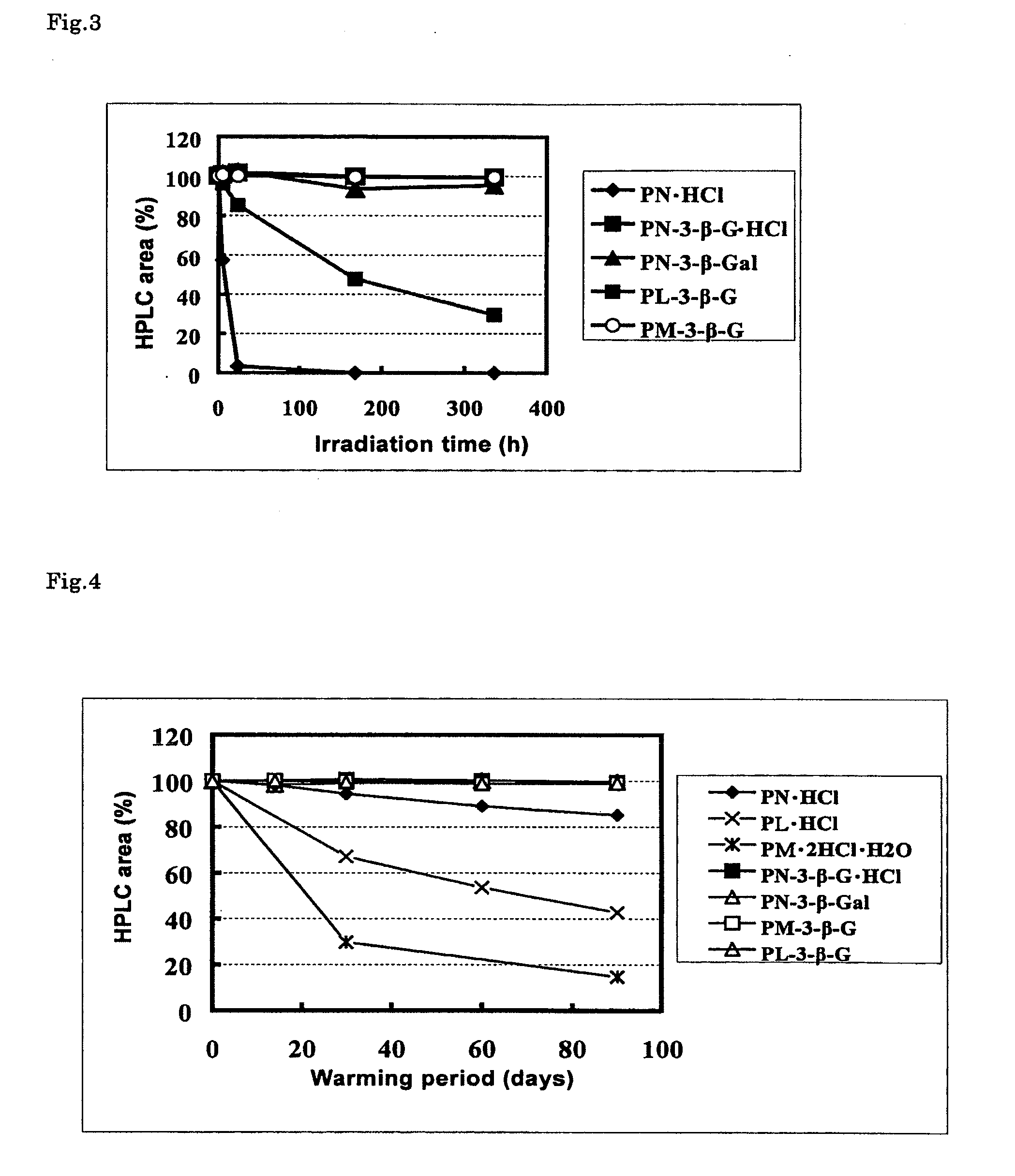
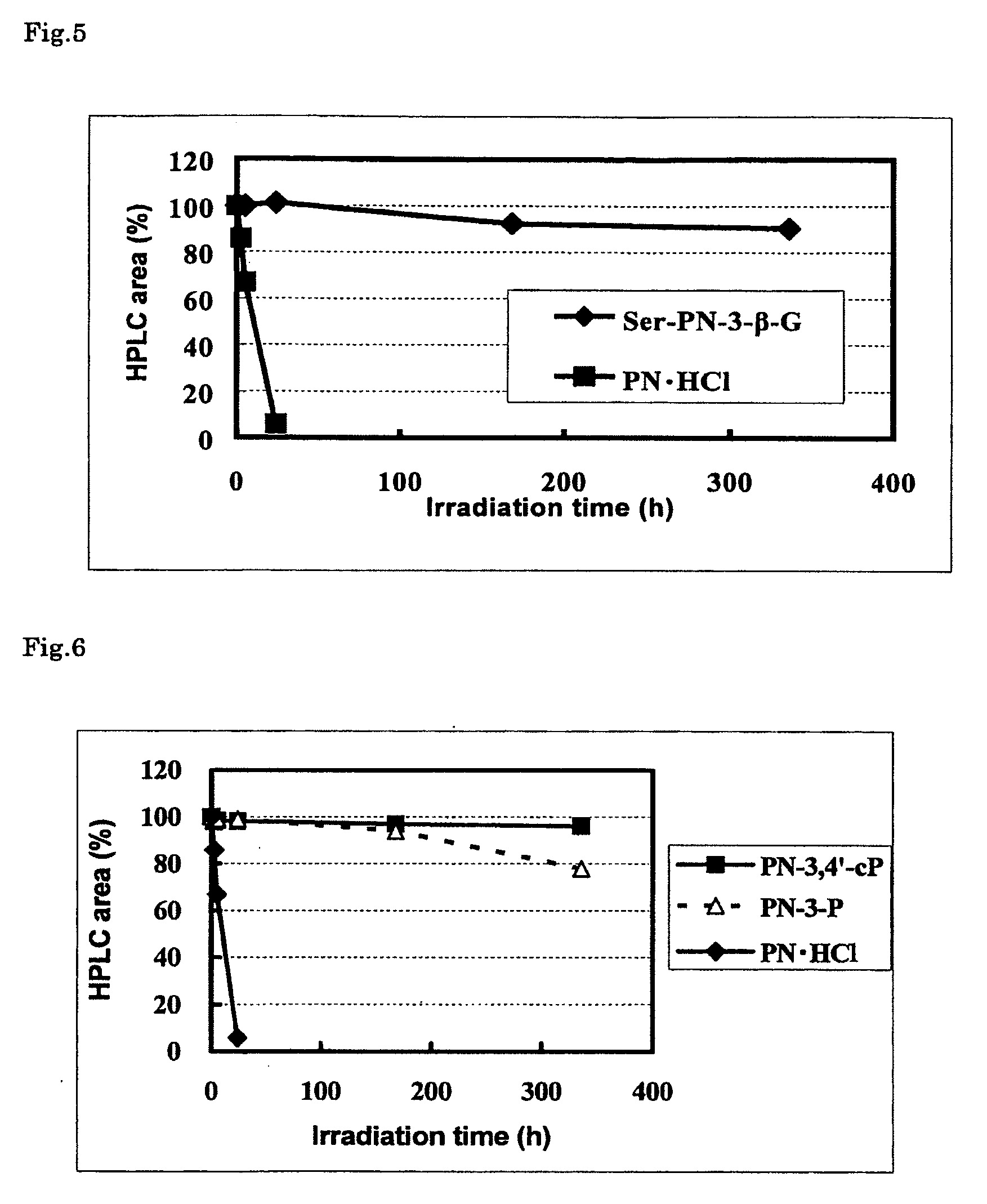
Stable vitamin b6 derivative
a technology of vitamin b6 and derivatives, applied in the field of stable vitamin b6 derivatives, can solve the problems of inability to light, obstacle to the practical use of these substances, etc., and achieve the effect of suppressing wrinkle formation and suppressing wrinkle formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Pyridoxine 3-β-D-glucoside
[0092]a) 3-(2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl)-α4, α5-di-O-acetylpyridoxine
[0093]α4, α5-Di-O-acetylpyridoxine hydrochloride (4.90 g, 17.2 mmol) was added with CHCl3 (150 ml) and saturated aqueous NaHCO3 (100 ml), and the mixture was stirred at room temperature for 1 hour. Then, the organic layer was washed with saturated brine, and dried over anhydrous MgSO4, and the solvent was evaporated under reduced pressure. The resulting white solid (4.0 g) and 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide (9.74 g, 23.7 mmol) were dissolved in CH2Cl2 (70 ml), and the solution was added with silver carbonate (4.36 g, 15.8 mmol) under light shielding and refluxed under a nitrogen atmosphere, and then stirred overnight. The reaction mixture was concentrated under reduced pressure, and then the residue was purified by column chromatography (silica gel, 600 g, eluted with a mixed solvent of n-hexane:ethyl acetate=1:2) to obtain the title compound...
example 2
Preparation of Pyridoxal 3-β-D-glucoside
[0097]a) 3-(2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl)-pyridoxal monoethylacetal
[0098]Pyridoxal monoethylacetal hydrochloride (11.0 g, 47.5 mmol) was suspended in CH2Cl2 (100 ml) under a nitrogen atmosphere, and added with triethylamine (6.63 ml, 47.5 mmol) under ice cooling, and the mixture was warmed to room temperature, and then added with 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide (23.4 g, 57.0 mmol). After the reaction vessel was light-shielded, the reaction mixture was added with silver carbonate (13.1 g, 47.5 mmol), and stirred at room temperature for 18 hours, and then stirring was continued at 35° C. for 24 hours. The reaction mixture was filtered, and concentrated under reduced pressure, and then the residue was purified by column chromatography (silica gel, 600 g, eluted with a mixed solvent of n-hexane:ethyl acetate=1:2) to obtain the title compound (20.8 g, 84%).
[0099]1H-NMR (CDCl3) δ ppm: 1.2-1.4 (3H, m), 2.0-2.1 (12H, m),...
example 3
Preparation of Pyridoxamine 3-β-D-glucoside
a) 3-(2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl)-pyridoxal Oxime
[0105]The compound of Example 2, b) (6.0 g, 12.1 mmol) was suspended in water (200 ml), and added with sodium acetate (1.29 g, 15.7 mmol) and hydroxylammonium chloride (1.26 g, 18.2 mmol), and the mixture was stirred for 30 minutes under reflux. The reaction mixture was cooled to room temperature, and then extracted with ethyl acetate (300 ml). The organic layer was dried over anhydrous MgSO4, and then the solvent was evaporated under reduced pressure. The residue was added with diethyl ether, and the deposited solid was collected by filtration, washed with diethyl ether, and then dried under reduced pressure to obtain the title compound (5.49 g, 89%).
[0106]1H-NMR (CDC3) δ ppm: 2.03 (3H, s), 2.03 (3H, s), 2.05 (3H, s), 2.19 (3H, s), 2.56 (3H, s), 3.5-3.7 (1H, m), 4.0-4.2 (2H, m), 4.61 (2H, brs), 4.80 (1H, d, J=7.9 Hz), 5.0 (1H, brs), 5.1-5.5 (3H, m), 8.40 (1H, s), 8.57 (1H, s),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| RH | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
| light stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com