Small Molecule Inhibitors of Toll-Like Receptor 9
a technology of toll-like receptor and inhibitor, which is applied in the field of small molecule inhibitors of toll-like receptor 9, can solve the problems of protective or adverse physiologic outcomes of the hos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Predicted Activities for Compounds of Formula III
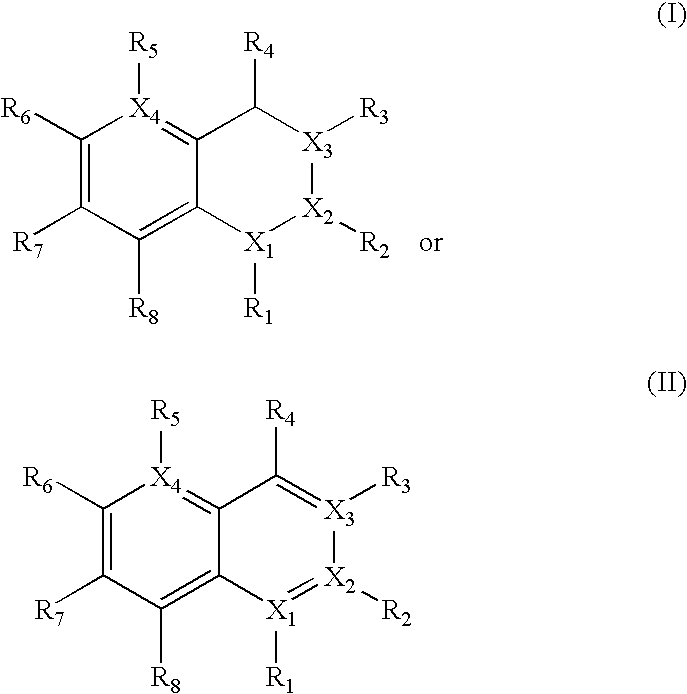
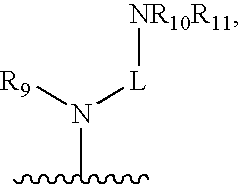
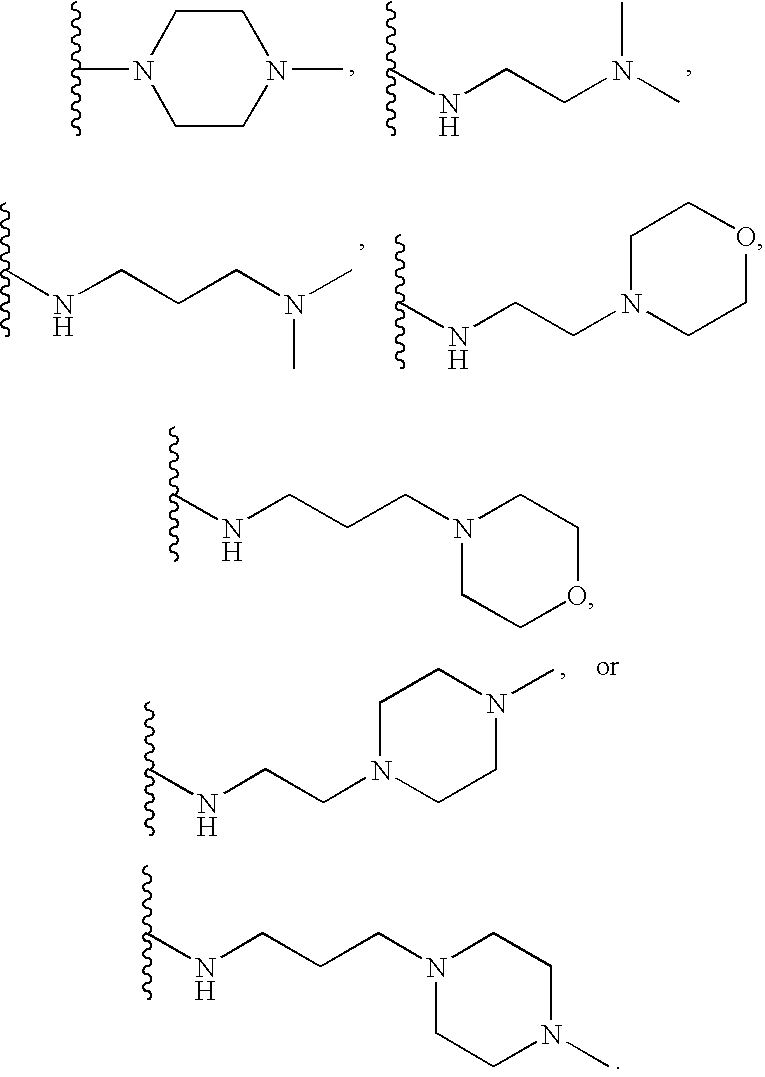
[0342]Based on computer modeling, IC50 values (nM) were predicted in respect of TLR9 activity for compounds according to Formula III wherein R3, R7, and R8 are hydrogen, and R6 is Yi (Ar—Y2). Substitutions for R4 and Y2 were made as shown in Table 1 below. In this set of data the compound with the lowest predicted IC50, 33 nM, had R4=dipip and Y2=dippip.
TABLE 1Y2pipdiaminedipaminedimordipmordipipdippipR4pip83594964485061diamine150564745644849dipamine591207475864879dimor66584150384158dipmor100585242414340dipip69413657395033dippip90463943584337
example 2
Predicted Activities for Compounds of Formula III
[0343]Based on computer modeling, IC50 values (nM) were predicted in respect of TLR9 activity for compounds according to Formula III wherein R3, R6, and R8 are hydrogen, and R7 is Y1 (Ar—Y2). Substitutions for R4 and Y2 were made as shown in Table 2 below. In this set of data the compound with the lowest predicted IC50, 33 nM, had R4=diamine and Y2=dippip.
TABLE 2Y2pipdiaminedipaminedimordipmordipipdippipR4pip67777675738491diamine791207965877533dipamine6867170908165110dimor65798283686636dipmor64759087797790dipip69558678666573dippip75737563728485
example 3
Predicted Activities for Compounds of Formula III
[0344]Based on computer modeling, IC50 values (nM) were predicted in respect of TLR9 activity for compounds according to Formula III wherein R3, R6, and R7 are hydrogen, and R8 is Y1 (Ar—Y2). Substitutions for R4 and Y2 were made as shown in Table 3 below. In this set of data the compound with the lowest predicted IC50, 51 nM, had R4=dimor and Y2=dipip.
TABLE 3Y2pipdiaminedipaminedimordipmordipipdippipR4pip530460120330440410160diamine100987866888382dipamine96888278769172dimor100647392775180dipmor7970771201306966dipip947568777811076dippip65675579607172
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com