Antibodies to egfl7 and methods for their use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
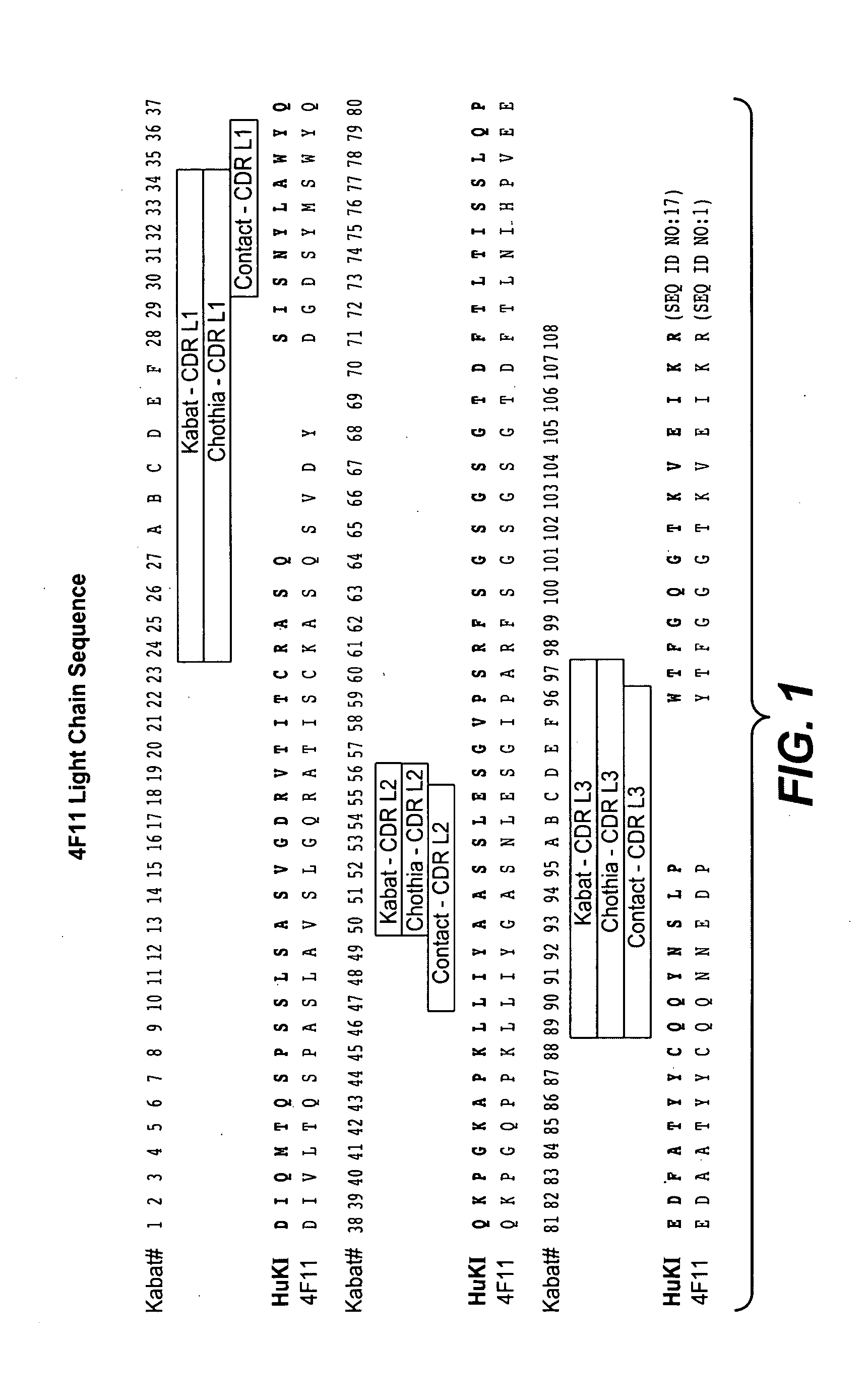
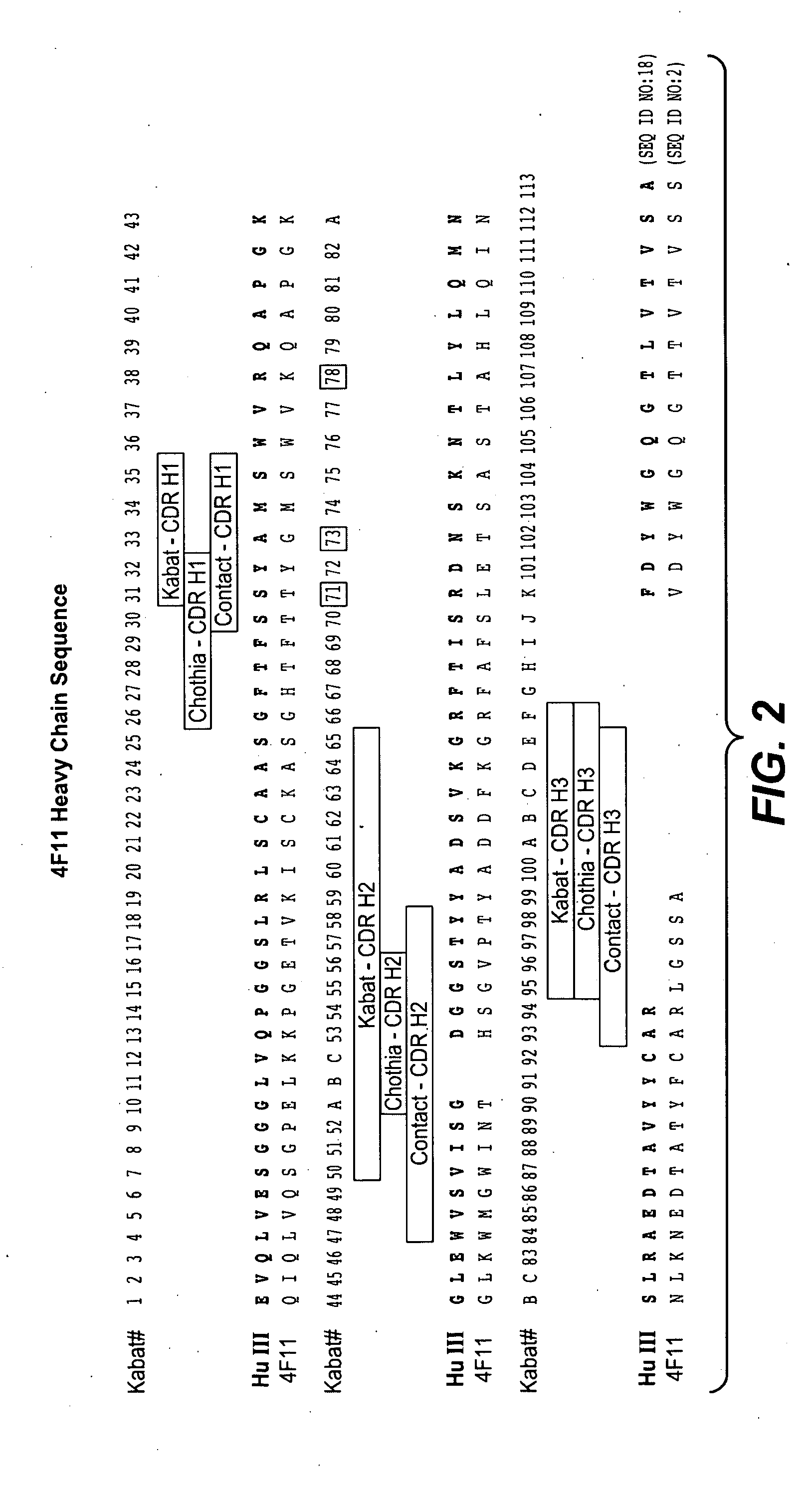
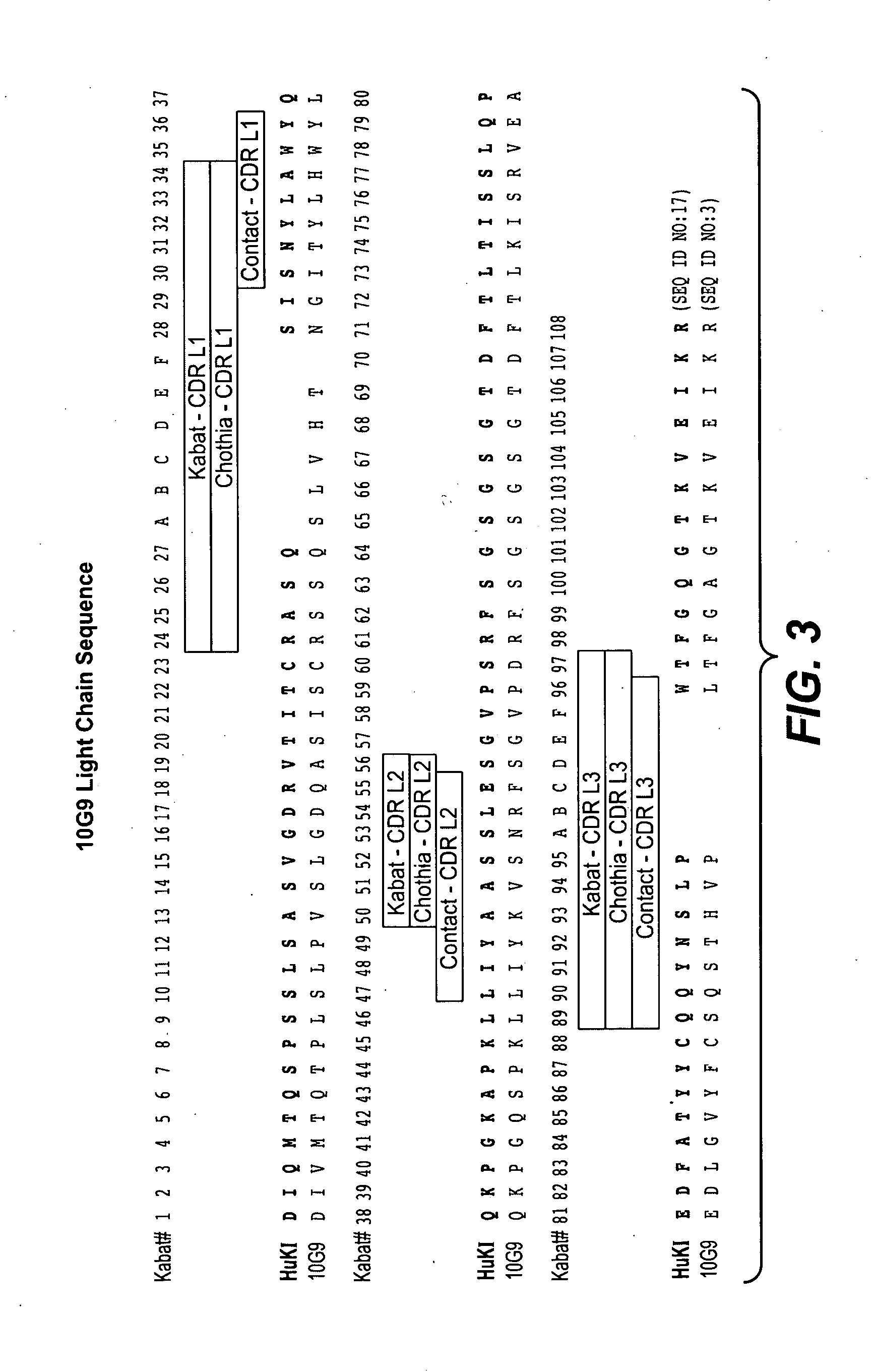
Production and Characterization of Monoclonal Antibodies to EGFL7
[0334]Production of Monoclonal Antibodies
[0335]EGFL7 was identified and cloned in an effort to discover novel human secreted and transmembrane proteins, particularly those involved in the regulation of vascular development. Details of the cloning and expression of human EGFL7 are described in, for example, patent application US2003 / 0224948A1 (in which EGFL7 is identified as PRO1449). The GenBank® accession number for human EGFL7 is NM—016215.
[0336]Egfl7 homozygous knockout mice (generated at Genentech) were immunized with E. coli produced His6-tagged recombinant human and mouse EGFL7 proteins, diluted in Ribi adjuvant (Corixia, Hamilton, Mont.) twice a week, via footpad, eight doses. B cells from lymph nodes were harvested from ten mice demonstrating high serum titers and were fused with mouse myeloma cells (X63.Ag8.653; American Type Culture Collection® (ATCC®)). After 10-14 days, the supernatants were screened for an...
example 2
Anti-EGFL7 Mabs Inhibit Tumor Growth In Vivo
[0351]In this example, anti-EGFL7 Mabs were tested for their ability to inhibit tumor growth in vivo in several models. We first tested the Mabs in PBS in the Colo205 model (human colorectal cancer) and the A673 model (human rhabdomyosarcoma model). We did not observe an effect in these models with the Mabs in PBS as single agents.
[0352]We then tested the Mabs alone and / or in combination with an anti-VEGF antibody, B20.4.1 (described in WO 2005 / 012359). We tested the antibodies in three models: a Her2 human breast cancer model (“Fo5 model”), a human lung cancer (NSCLC) model (“H1299”) and another human breast cancer model (“MDA-MB231”). These tumor models are well established and are described in, e.g., Lee et al., Clin Cancer Res. 11(16):6065-74 (2005); Cameron et al., Cancer Cell Int.5:23 (2005); Finkle et al., Clinical Cancer Res. 10:2499-251 (2004). Each animal was treated with anti-ragweed Mab (control); B20.4 alone; 18F7 alone; or B2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Chemotherapeutic properties | aaaaa | aaaaa |
| Antigenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com