A process for synthesising silver nanoparticles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Example
Example 1
Microfluidic Production of Silver Seeds
[0170]We have found that by using microfluidic technologies for the production of silver seeds control over the synthesis of the silver seeds is an important factor in producing discrete high definition silver nanoparticles with predetermined, size, shape and a narrow distribution of size and shape
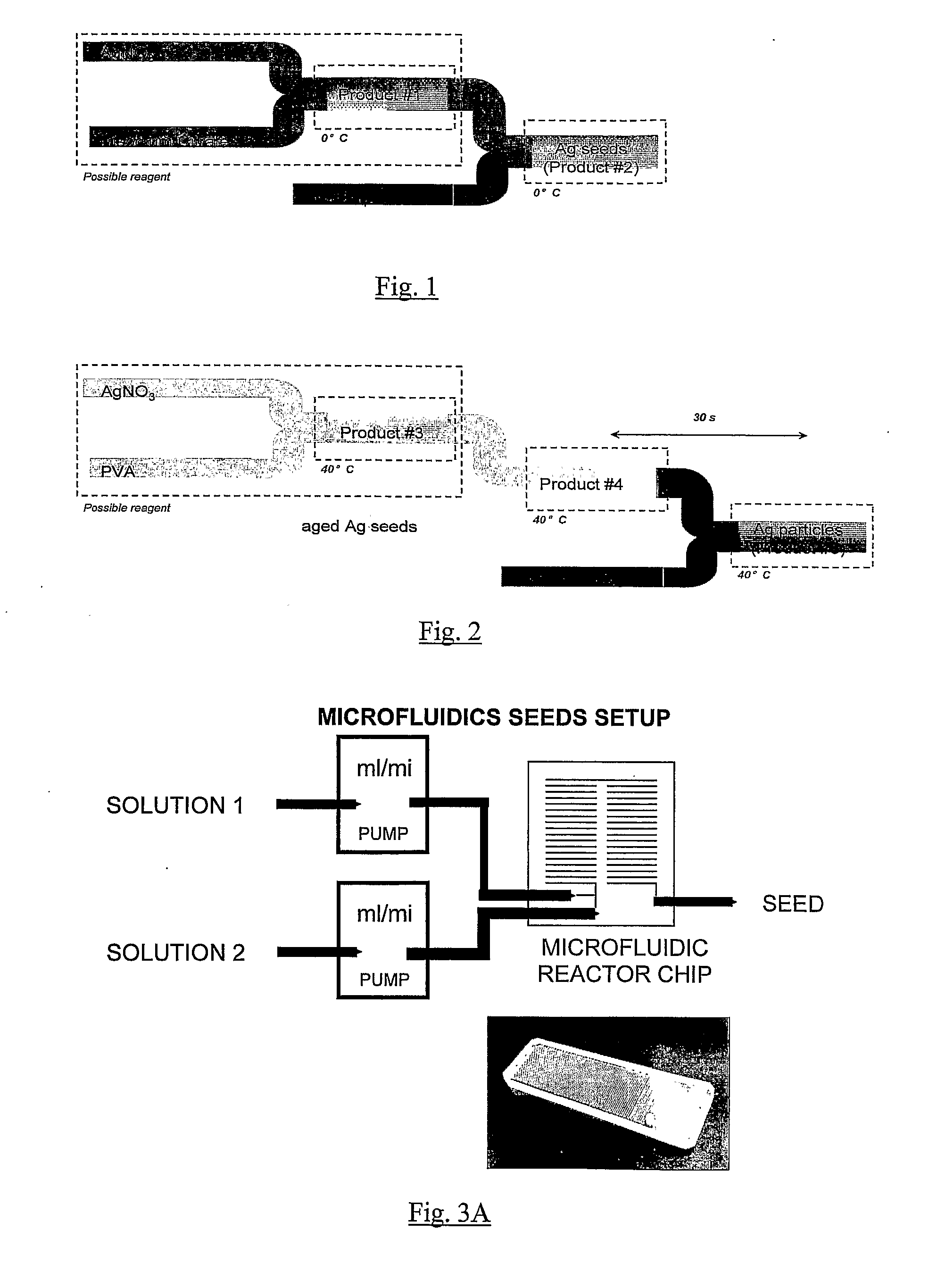
[0171]FIG. 1 is a schematic illustrating the set up for microfluidic synthesis of silver seeds.
[0172]The constituent chemicals and products may vary from those detailed in FIG. 1 wherein product 1 is a silver nitrate (AgNO3) and Trisodium Citrate (TSC) solution and product 2 is a sodium borohydride (NaBH4) solution. Briefly, referring to FIG. 1, a silver source (in this case silver nitrate) is mixed with trisodium citrate at about 0° C. Following mixing sodium borohydride (NaBH4) is added to the AgNO3-TSC solution and the mixture is incubated at about 0° C.
[0173]In an alternative process, product 1 is a sodium borohydride (NaBH4) and Trisodiu...
Example
Example 2
Protocol for the Production of Silver Seeds Using a Microfluidic Chip System
[0175]Referring to FIGS. 3A and 4, dissolve 37.8 mg of sodium borohydride in 100 ml of water (Solution 1 of FIG. 3A).
[0176]Dissolve 5 mg of silver nitrate and 7.4 mg of trisodium citrate in 100 ml of iced cooled water in an ice bath (solution 2 of FIG. 3A).
[0177]Connect solution 1 and solution 2 to pump 1 and pump 2 respectively (see setup of FIGS. 3A and 4).
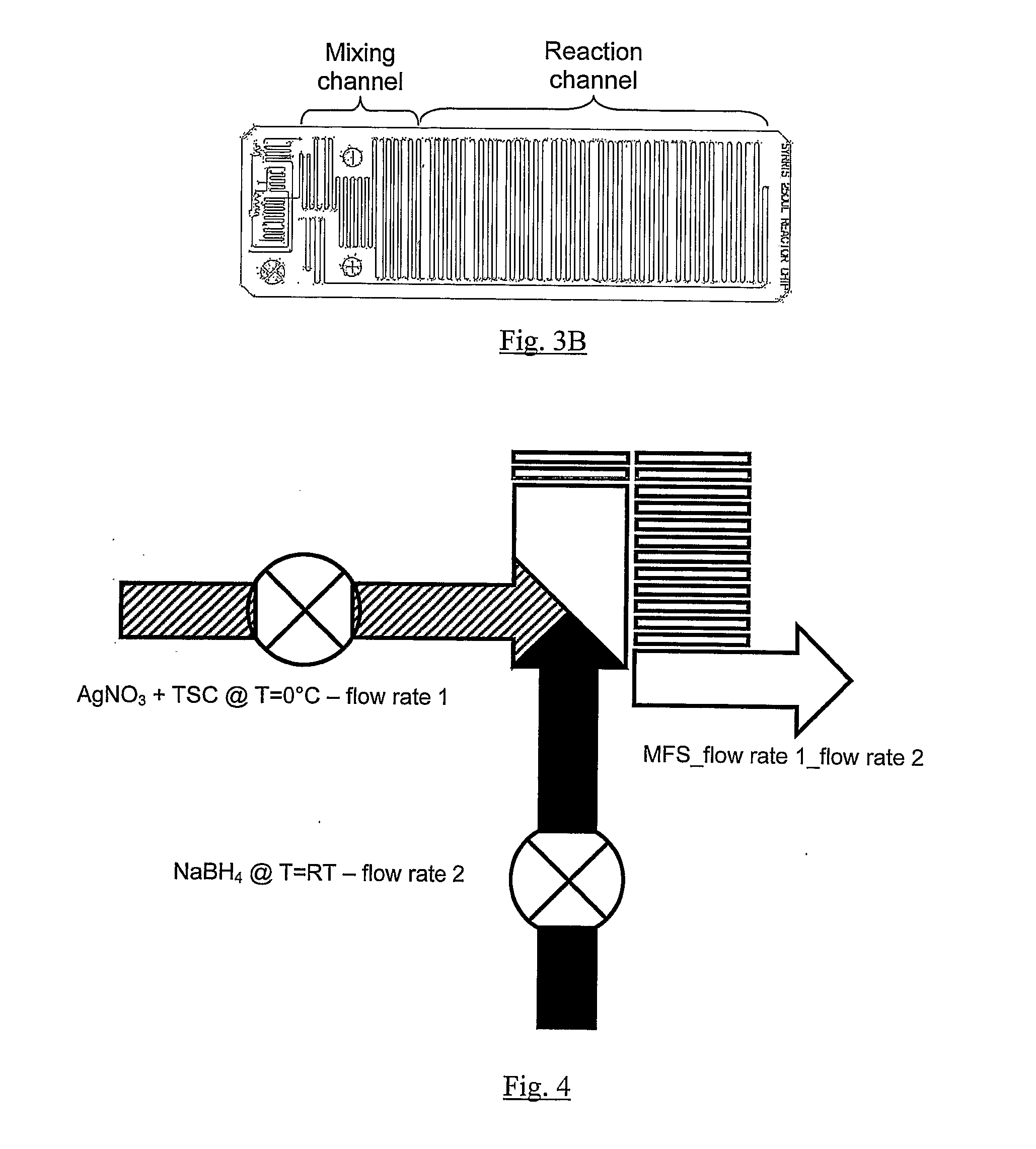
[0178]Set pump 1 and pump 2 flow rates for example at 1 ml / min and 8 ml / min respectively;[0179]Run pump 1 for 30 s and collect by-product;
[0180]Run pump 2, while pump 1 is still running and collect by-product for 30 s.
[0181]Collect 5 ml of final seed product, while pump 1 and pump 2 are still running.
[0182]Stop both pump 1 and pump 2.
[0183]A more generic setup for reagent input sequencing for general nanoparticle production is shown in FIG. 5. This setup can be applied to the production method for of a wide range of nanoparticles including high ...
Example
Example 3
Experimental Results for Application of Microfluidics Methods to Silver Seed Production
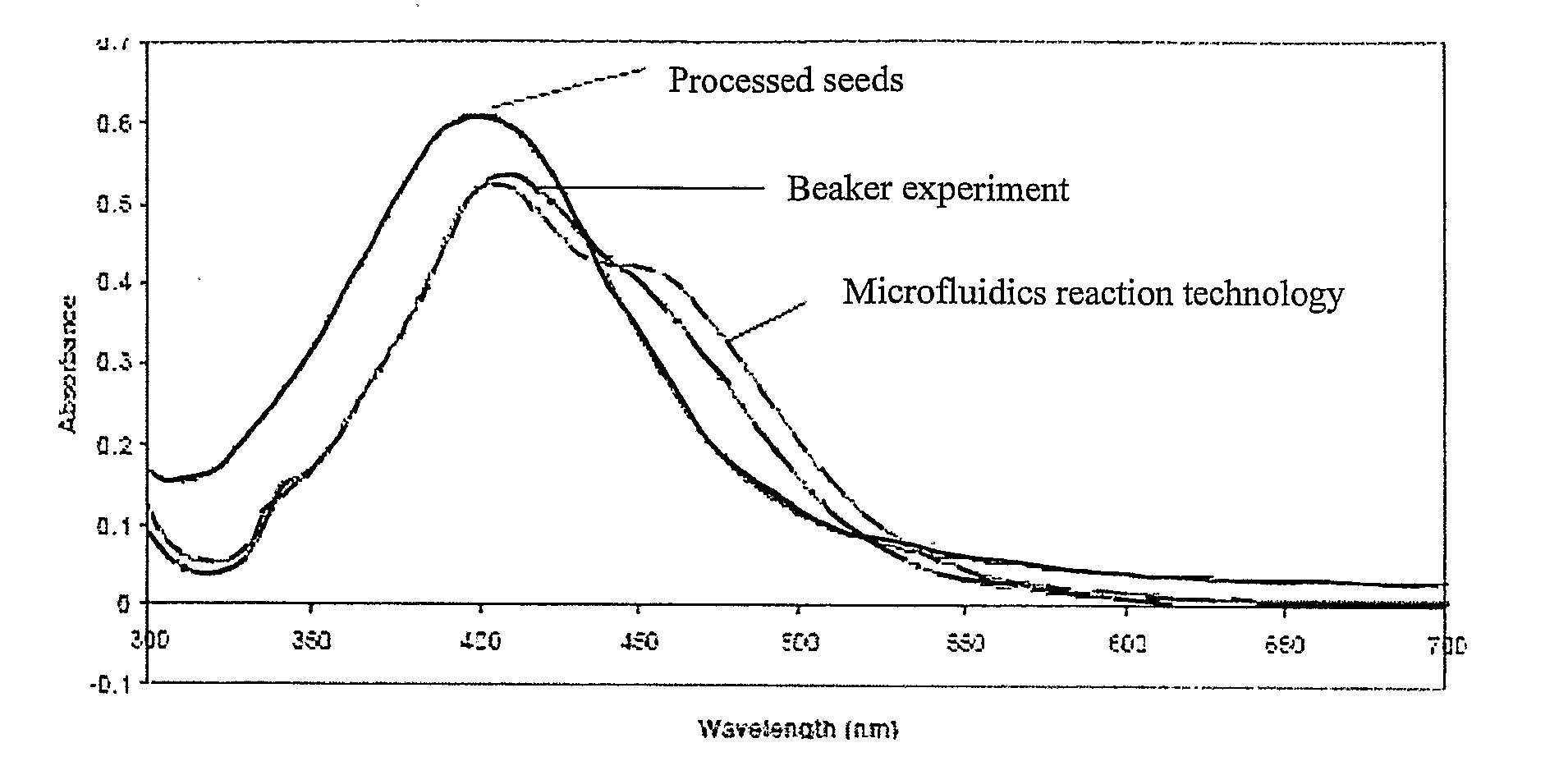
[0184]The results from experiments using a generic microfluidic chip system for the production of silver seeds (step (a)) are given below. In these cases the second step, (the growth of these seeds to produce discrete high definition silver nanoparticles) was carried out using a conventional batch chemistry method.
[0185]In this example, silver seeds were synthesised using a generic microfluidic chip system according to the following method:
[0186]37.8 mg of sodium borohydride (0.01M) was dissolved in 100 ml of water (solution 1 of FIG. 3A). 5 mg of silver nitrate (2.94×10−4M) and 7.4 mg of trisodium citrate (2.5×10−4M) were dissolved in 100 ml of iced cooled water in an ice bath (solution 2 of FIG. 3A). Solution 1 and solution 2 were connected to pump 1 and pump 2 respectively (as shown in the setup of FIGS. 3A and 4). The flow rate of pump 1 was set at 1 ml / min under a pressure of about 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pressure | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com