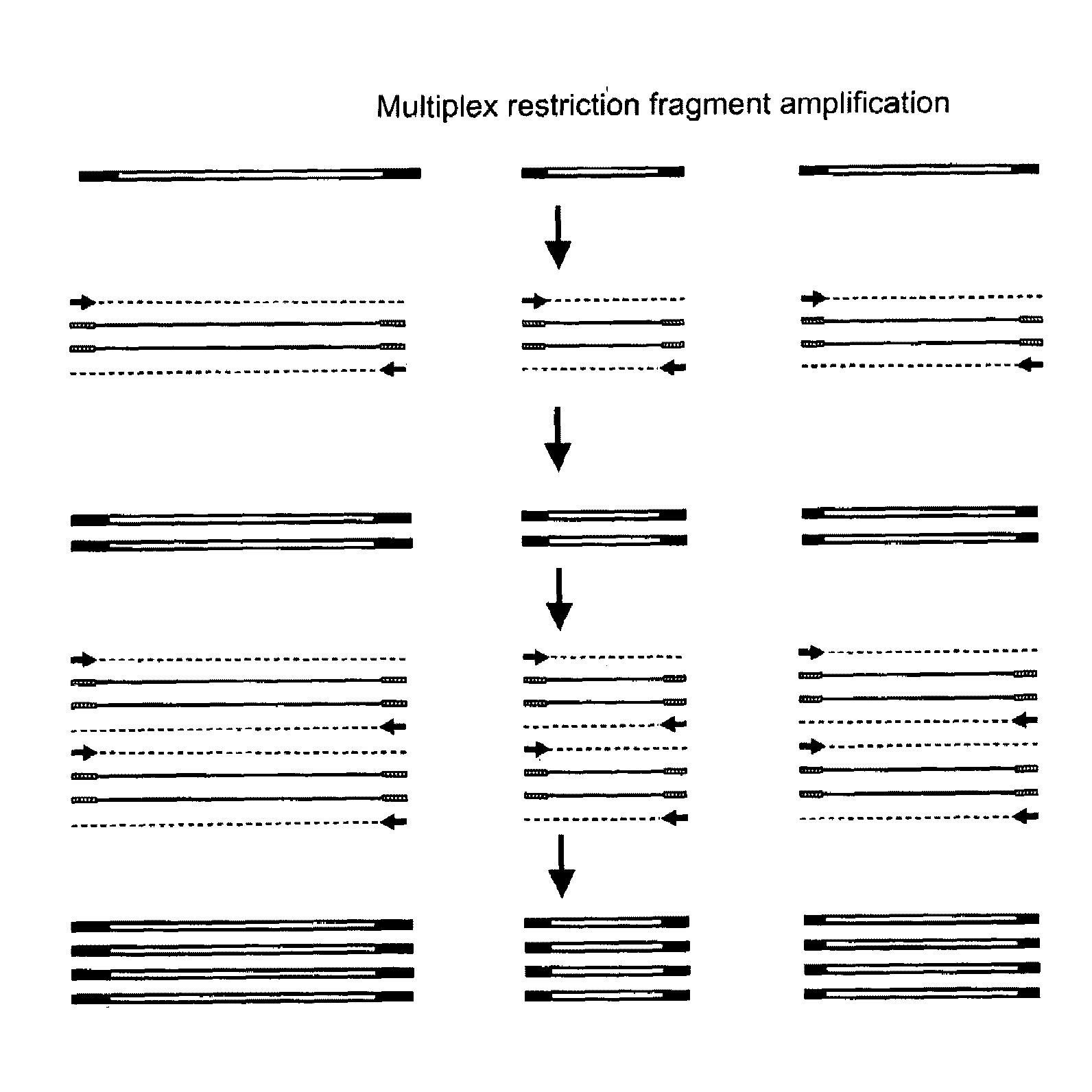
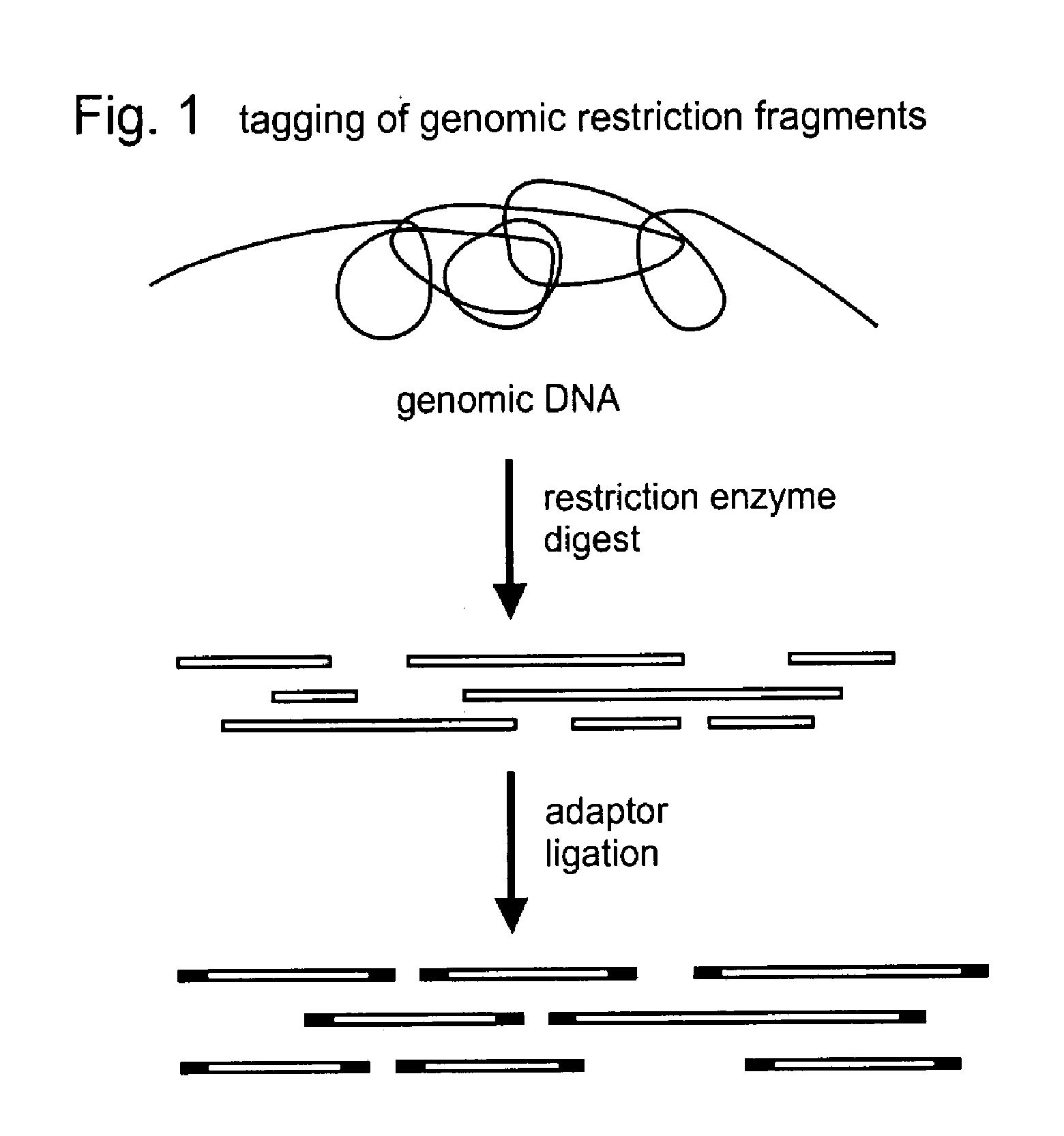
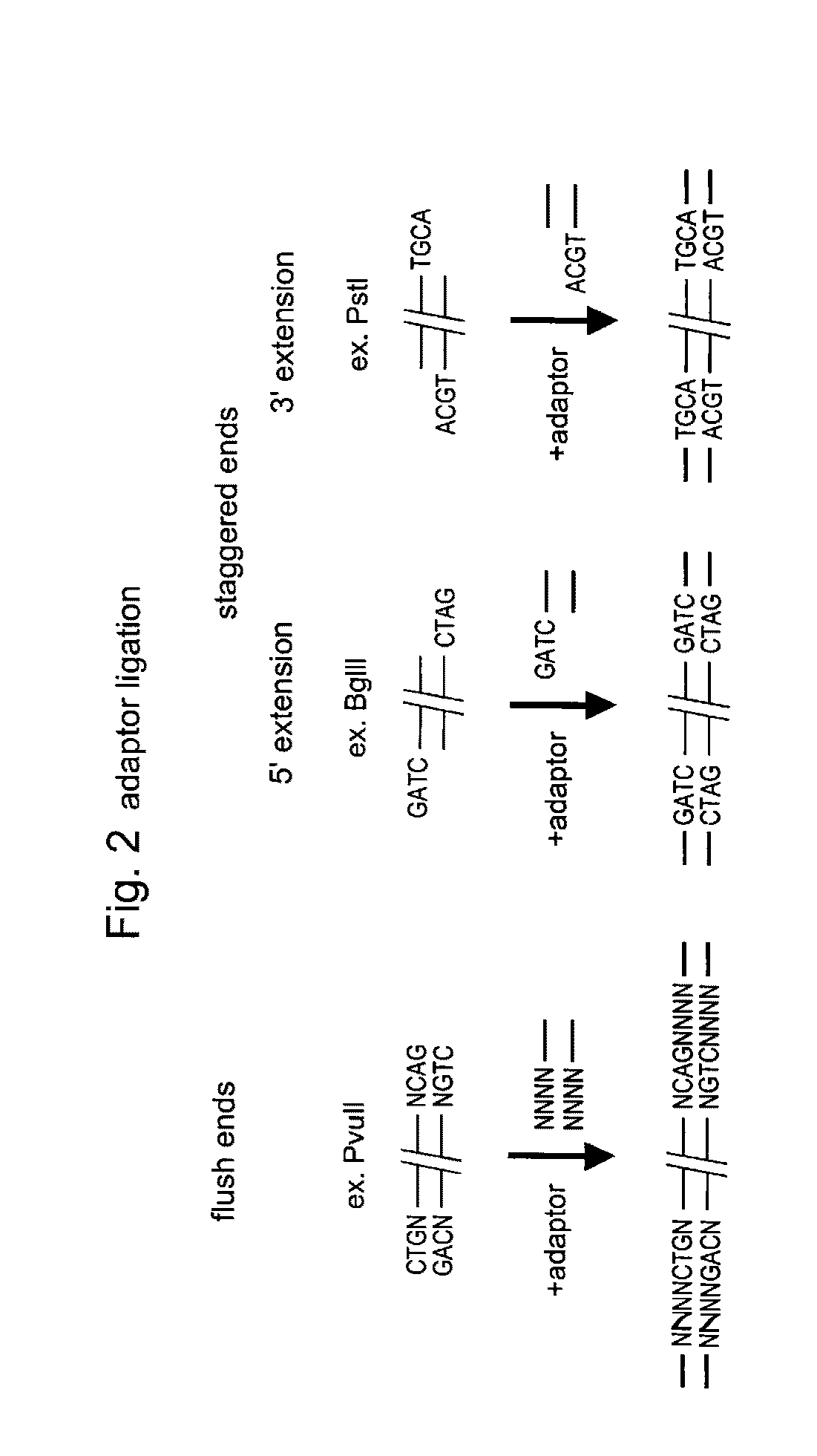
Selective restriction fragment amplification: fingerprinting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Selective Restriction Fragment Amplification of Tomato DNA Using PstI
A) Isolation and Modification of the DNA
[0081]Total Tomato DNA (Lycopersicon esculentum c.v. Moneymaker) was isolated from young leaves as described by Bernatzski and Tanksley (Theor. Appl. Genet. 72, 314-321). The typical yield was 50-100 μg DNA per gram of fresh leaf material. The DNA was restricted with PstI (Pharmacia) and double-stranded (ds) PstI-adapters were ligated to the restriction fragments following the procedure described below. These adapters had the following structure (SEQ ID NOS:1-2):
5- CTCCTAGACTGCGTACATGCA -33- CATCTGACGCATGT -5
The 3′TGCA-overhang in these adapters anneals to the staggered ends created by PstI. The PstI recognition sequence CTGCAG is not restored upon ligation of this adapter, because the 5′ C-residue is replaced by A. The ligation reaction was designed in such a way that the end result is almost exclusively DNA fragment-to-adapter molecules. This was achieved by: 1. using non-p...
example 2
Selective Restriction Fragment Amplification of Tomato DNA with Two Restriction Enzymes
[0101]In example 1 the principle of selective restriction fragment amplification (SRDA) is exemplified using Tomato DNA and the restriction enzyme PstI. In this example SRFA using two different restriction enzymes, PstI and MseI, will be illustrated.
Isolation and Modification of the DNA
[0102]Total Tomato DNA was isolated from young leaves as described in example 1. Two pairs of so called isogenic lines were used as source of the DNA, name GemR and GemS, and CGR26 and GCR151 respectively (These lines are described in the following references: Denby and Williams, (1962), Can. J. Plant Sci. 42, 601-685, Smith and Ritchie, (1983), Plant Mol. Biol. Rep. 1, 41-45). The two individuals of each pair of isogenic lines are genetically very similar, but differ in the presence of a trait confering resistance to the fungal pathogen Verticuillium albo-atratum.
[0103]The first step of the modification of the DNA...
example 3
Selective Restriction Fragment Amplification of DNA of Various Lactuca Species with Two Restriction Enzymes
[0131]In example 2 the principle of selective restriction fragment (SRFA) amplification using two restriction enzymes is exemplified for Tomato DNA. In this example we will illustrate that similar results are obtained using DNAs of various Lactuca species using the same two restriction enzymes PstI and MseI.
Isolation and Modification of the DNA
[0132]DNAs were isolated as described in example 1 using young leaf material of various Lactuca species. As indicated below these plants include a commercial lettuce (L. sativa) variety, and several individuals of two wild Lactuca species, L. saligna and L. virosa. The plants were arbitrarily designated the following names:
1. L. saligna, nr. 21, plant 1
2. L. saligna, nr. 21, plant 2
3. L. saligna, nr. 22, plant 1
4. L. saligna, nr. 22, plant 2
5. L. virosa, nr. 01, plant 1
6. L. virosa, nr. 01, plant 2
7. L. virosa, nr. 02,
8. L. virosa, nr. 03...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com