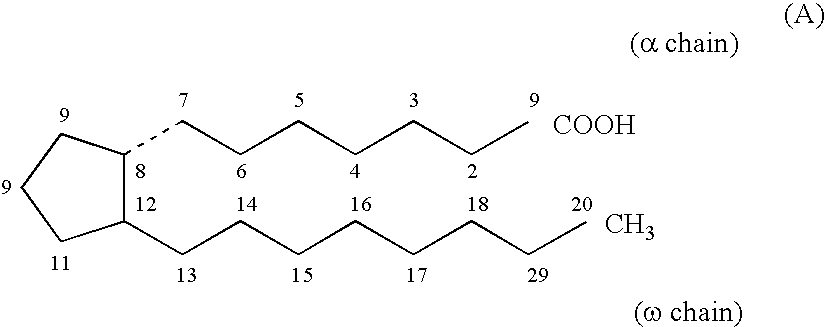
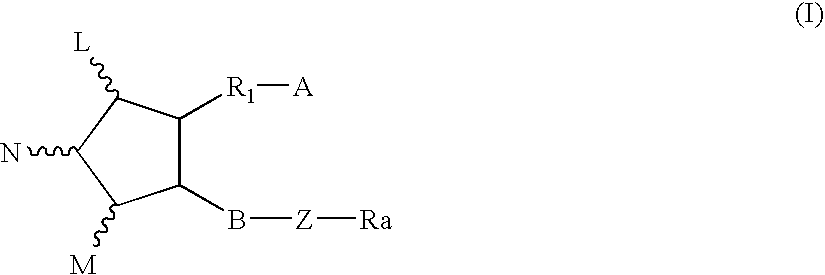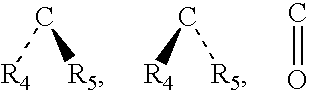Method for treating abdominal discomfort
a chloride channel and abdominal discomfort technology, applied in the field of prostaglandin, can solve the problems of abdominal discomfort, abdominal discomfort may also occur, not known as to drugs, etc., and achieve the effect of significant effect on abdominal discomfor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methods
[0125]Patients with irritable bowel syndrome (IBS) were randomly allocated to the following two treatment groups.[0126]Group 1: Test substance (13,14-dihydro-15-keto-16,16-difluoro-PGE1) 48 μg total (24 μg / breakfast+24 μg / dinner)[0127]Group 2: Matching placebo (placebo / breakfast+placebo / dinner)
[0128]Each group underwent two weeks washout period and then began to administer oral test substance (capsules) or placebo (capsules) daily for 4 weeks. Test substance or placebo was taken two times a day (b.i.d) at breakfast with food and at least 8 ounces of water and at dinner with food and at least 8 ounces of water. Patients were asked to evaluate abdominal discomfort upon waking in the morning, using a 5-point scale (Score: 0=absent, 1=mild, 2=moderate, 3=severe, 4=very severe) at 4 weeks after the initiation of the treatments.
[0129](Results)
[0130]As shown in Table 1, test substance of this invention significantly improved the abdominal discomfort in the patients with IBS.
TABLE 1E...
example 2
Methods
[0131]Patients with occasional constipation were randomly allocated to the following two treatment groups.[0132]Group 1: Test substance (13,14-dihydro-15-keto-16,16-difluoro-PGE1) 48 μg total (24 μg / breakfast+24 μg / dinner)[0133]Group 2: Matching placebo (placebo / breakfast+placebo / dinner)
[0134]Each group underwent two weeks washout period and then began to administer oral test substance (capsules) or placebo (capsules) daily for 4 weeks. During the washout period, the patient's bowel habit was documented to confirm the existence of constipation. Constipation is defined as, on average, less than three spontaneous bowel movements per week. All existing laxative medication was withdrawn at the start of the washout period and the patients were instructed not to change their diet or lifestyle during the study.
[0135]Test substance or placebo was taken orally for a total treatment period of 4 weeks; it was taken two times a day (b.i.d) at breakfast with food and at least 8 ounces of ...
example 3
Methods
[0139]Patients with irritable bowel syndrome (IBS) were randomly allocated to the following two treatment groups.[0140]Group 1: Test substance (13,14-dihydro-15-keto-16,16-difluoro-PGE1) 48 μg total (24 μg / breakfast+24 μg / dinner)[0141]Group 2: Matching placebo (placebo / breakfast+placebo / dinner)
[0142]Each group underwent two weeks washout period and then began to administer oral test substance (capsules) or placebo (capsules) daily for 4 weeks. Test substance or placebo was taken two times a day (b.i.d) at breakfast with food and at least 8 ounces of water and at dinner with food and at least 8 ounces of water. The patients were asked to evaluate abdominal bloating upon waking in the morning, using a 5-point scale (Score: 0=absent, 1=mild, 2=moderate, 3=severe, 4=very severe) at 4 weeks after the initiation of the treatments.
[0143](Results)
[0144]As shown in Table 3, test substance of this invention significantly improved the abdominal bloating in patients with IBS.
TABLE 3Effec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| Stress | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com