Treatment of Cancers Expressing p95 ErbB2
a cancer and erbb2 technology, applied in the field of solid tumor treatment, can solve the problems of poor response to therapy, damage or kill normal cells, and many existing anti-cancer chemotherapeutics are non-specifi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Materials
HN5, an EGFR overexpressing LICR-LON-HN5 head and neck carcinoma cell line was kindly provided by Helmout Modjtahedi at the Institute of Cancer Research, Surrey, U.K. The ErbB2 overexpressing human breast adenocarcinoma cell line, BT474 was obtained from the American Type Culture Collection (Manassas, Va., USA). HB4a cells are derived from human mammary luminal tissue; ErbB2 transfection of parental HB4a cells yielded the HB4a C5.2 cell line (Harris et al., Int J. Cancer. 80:477 1999). 51 cells, which express elevated levels of p-ErbB2 were established by sub-cloning HB4a C5.2 (Xia et al., Oncogene, 21:6255 (2002)). EGF was purchased from Sigma Chemical (St. Louis, Mo., USA). Recombinant human NRG-1-B1 / HRGB1 EGFR domain (heregulin, HRG) was from RD system (Minneapolis, Minn., USA). Anti-phosphotyrosine antibody was purchased from Sigma and Upstate (Lake Placid, N.Y., USA). Anti-EGFR (Ab-12) and anti-c-ErbB2 (Ab-11) monoclonal antibodies were from Neo Ma...
example 2
Inhibition of p95 by GW572016
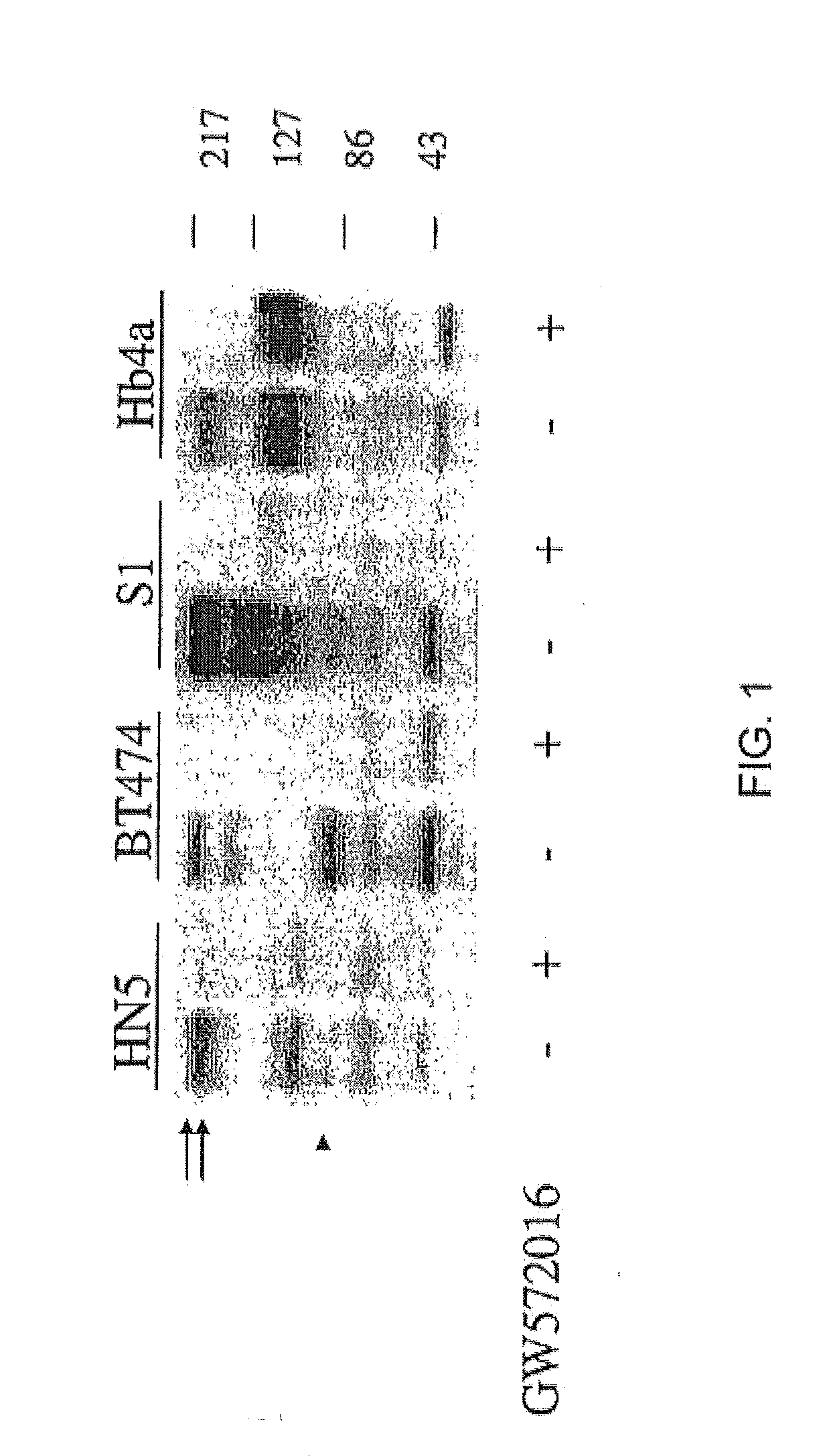
FIG. 1 demonstrates the effects of GW572016 on the expression of phospho-ErbB2 (p185), EGFR (p170), and p95 in BT474, HN5, 51 and Hb4a cell lines. Western blot analysis was performed using equal amounts of protein from whole cell extracts using anti-pTyr monoclonal antibody. Steady state protein levels of phosphorylated p185ErbB2 (top arrow), p170EGFR (lower arrow) and p95 (arrowhead) are shown in FIG. 1. Cells were treated with vehicle alone (DMSO at a final concentration of 0.1%; indicated by “−”) or GW572016 (1 μM for BT474; 5 μM for other cell lines, indicated by “+”) for 24 hours.
As shown in FIG. 1 (compare lanes 3 and 4), treating ErbB2 overexpressing BT474 breast cancer cells with 1 μM GW572016 inhibited not only p185ErbB2 phosphorylation (top arrow) but also inhibited a 95 kDa phosphotyrosine protein (p95, arrowhead).
In S1 cells, treatment with 5 μM GW572016 inhibited p185ErbB2 and p95 phosphorylation (FIG. 1, compare lanes 5 and 6). S1 cells are...
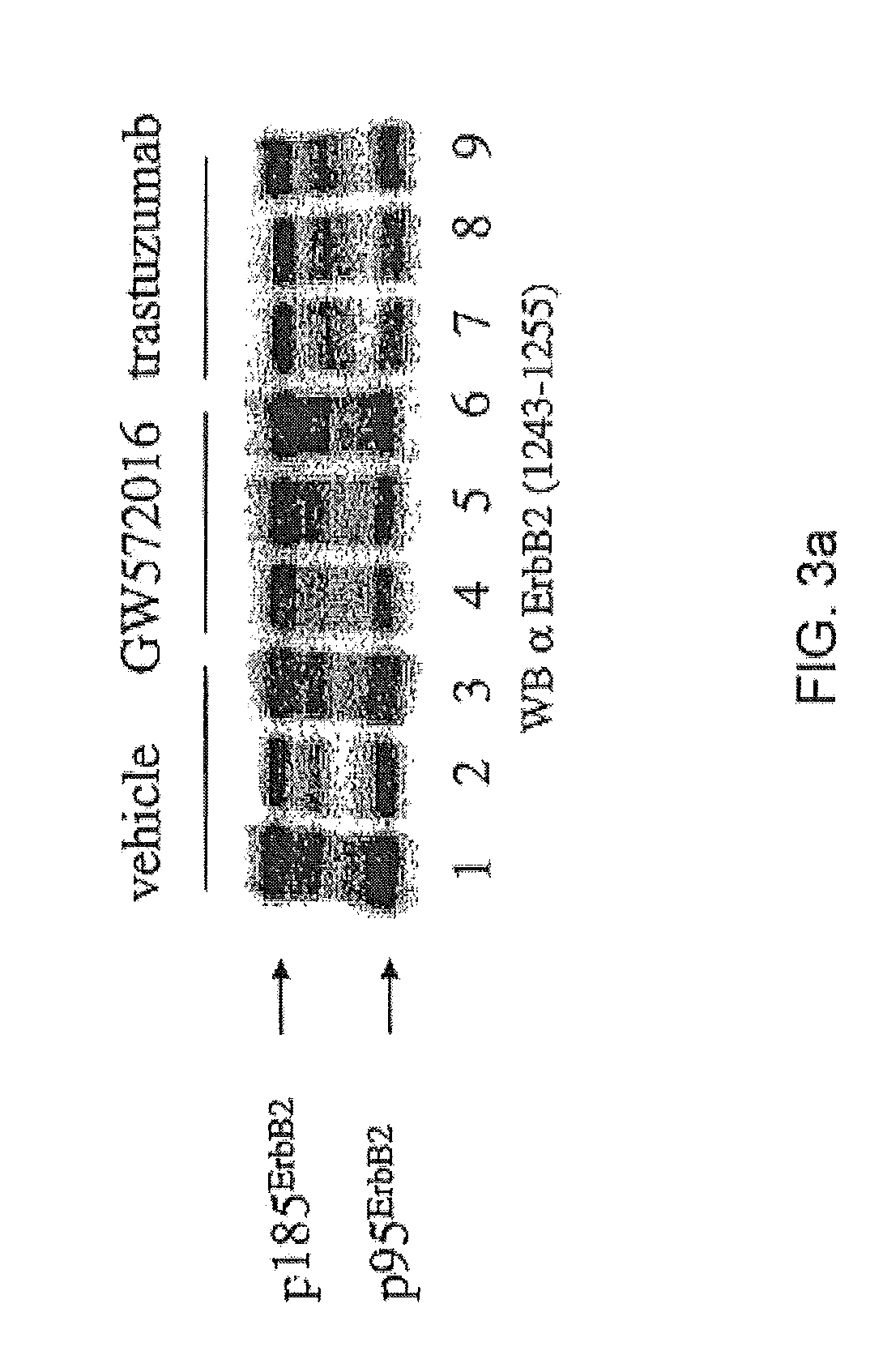
example 3
Identification of p95 as the Truncated ErbB2 Receptor (p95ErbB2)
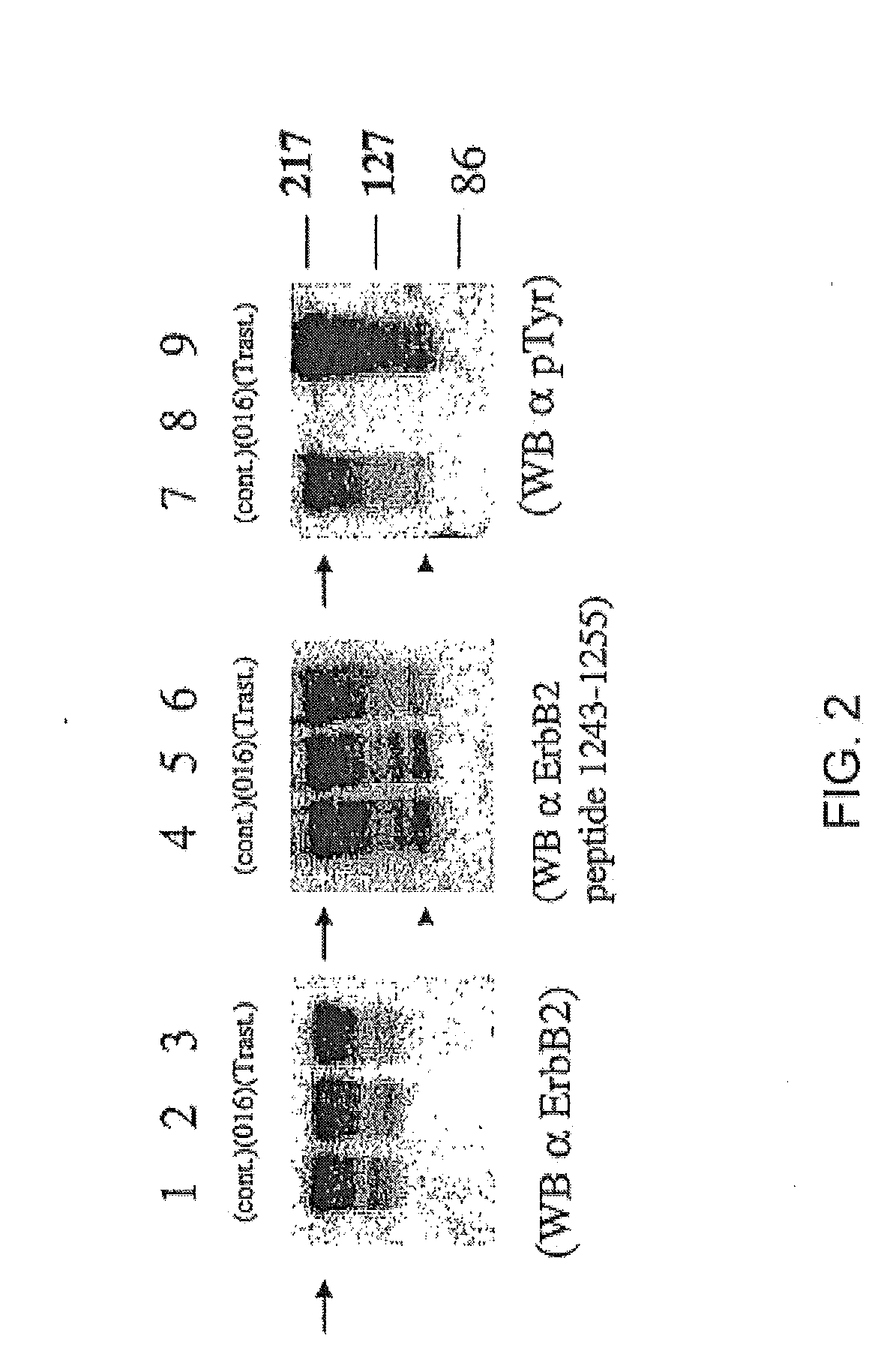
Proteolytic cleavage of the extracellular domain of p185ErbB2 leads to the appearance of the truncated 95 kDa ErbB2 receptor (p95ErbB2), which is highly phosphorylated (Christianson et al., Cancer Res. 58:5123 (1998)). To determine whether the 95 kDa phosphotyrosine protein as identified in the Example 1 was p95ErbB2, equal amounts of protein from total cell extracts were subjected to Western blot analysis using anti-ErbB2 mAbs that recognized distinct epitopes.
As shown in FIG. 2, exponentially growing BT474 cells were co-cultured for 24 hours with vehicle alone (0.1% DMSO; lanes 1, 4 and 7), 0.5 μM GW572016 (lanes 2, 5 and 8), or 10 μg / ml trastuzumab (lanes 3, 6 and 9). Equal amounts of protein were separated by SDS-PAGE and then ErbB2, p95ErbB2, pTyr / p95ErbB2, and pTyr / ErbB2 steady state protein levels were assessed by Western blot. Blots were probed with the following monoclonal antibodies: (a) anti-ErbB2 ECD (lanes ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
| Level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com