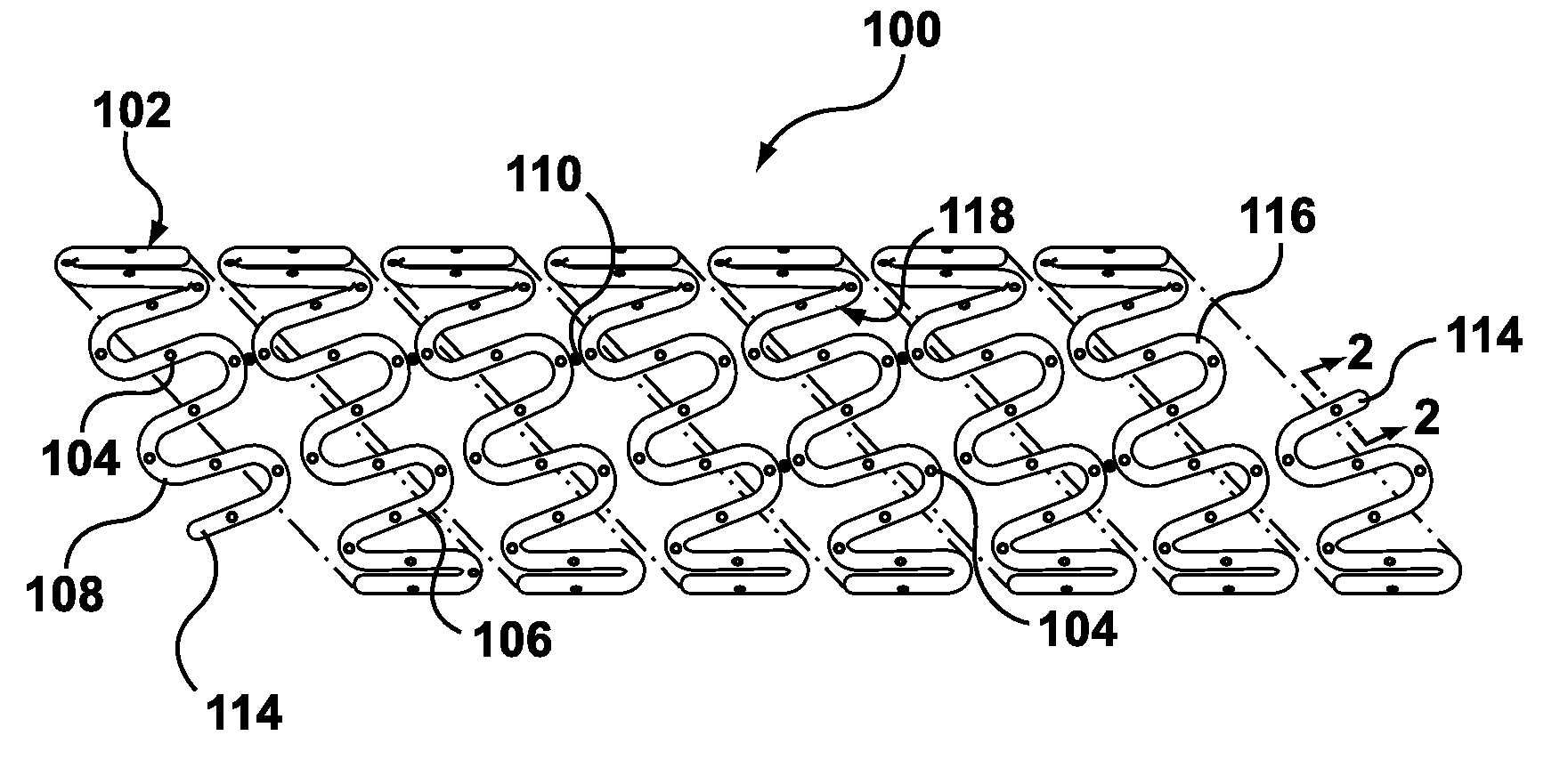
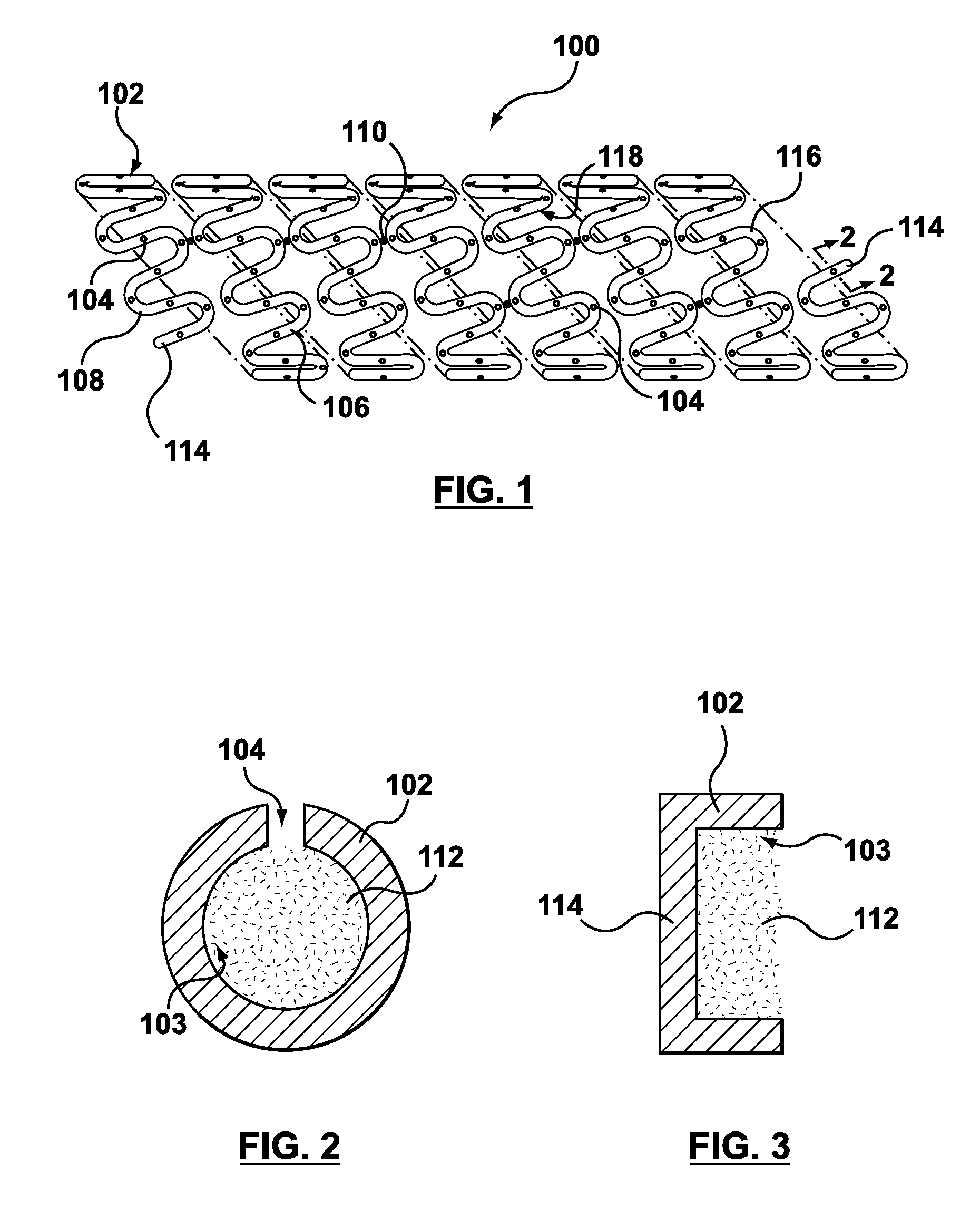
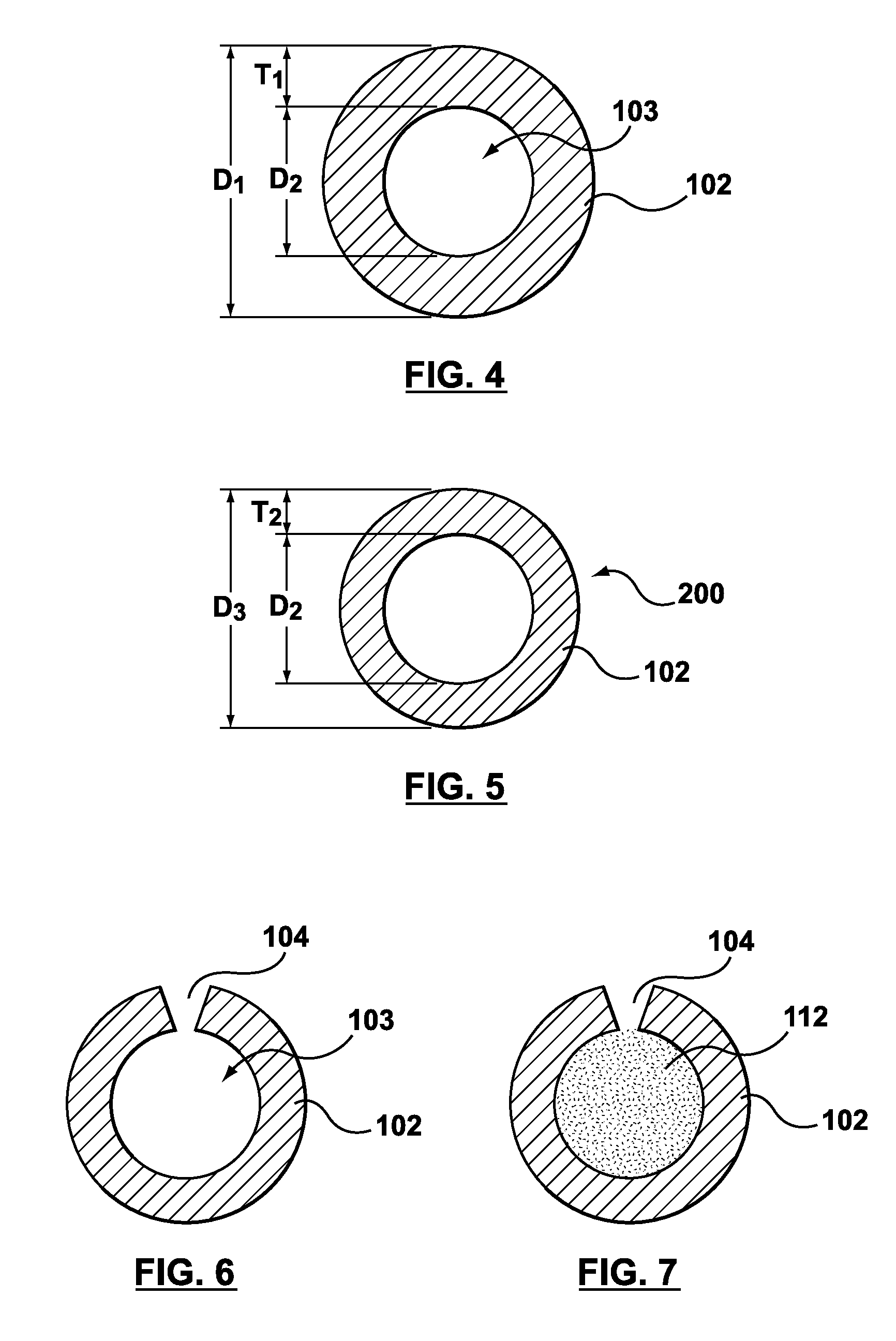
Method of forming hollow tubular drug eluting medical devices
a technology of medical devices and hollow tubules, applied in the field of implantable medical devices, can solve the problems of small but dangerous clot formation, biological or pharmacologically active substances not reaching the target site, and limited drug impregnation polymer coatings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
embodiment
Sheet Embodiment
[0055]Another method for forming a stent including hollow struts is described referring to FIGS. 26-30. In this embodiment, rather than forming the stent from a wire bent into a stent pattern, the stent pattern is cut into flat sheets, rolled into a tube, and welded to form a stent with hollow struts. As illustrated schematically in FIG. 26, a first sheet 402 and second sheet 404 sandwich a third sheet 406 therebetween. First and second sheets 402, 404 are made from materials that form a finished stent, such as stainless steel, nitinol, MP35N, etc. Third sheet 406 is made from a material that can be removed from between first and second sheets 402, 404 after forming. For example, the materials described in co-pending U.S. application Ser. No. 12 / 500,359, filed Jul. 9, 2009 and incorporated by reference herein, for the outer member can be used for first and second sheets 402, 404, and the materials described therein for the sacrificial or core member can be used as th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
| Melt temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com