Angiohematopoietic Progenitor Cells
a technology of angiohematopoietic progenitor cells and angiohematopoietic stem cells, which is applied in the field of angiohematopoietic stem cells, can solve the problems of limited utility of human psc cultures and significant challenge in hpsc culture identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Introduction
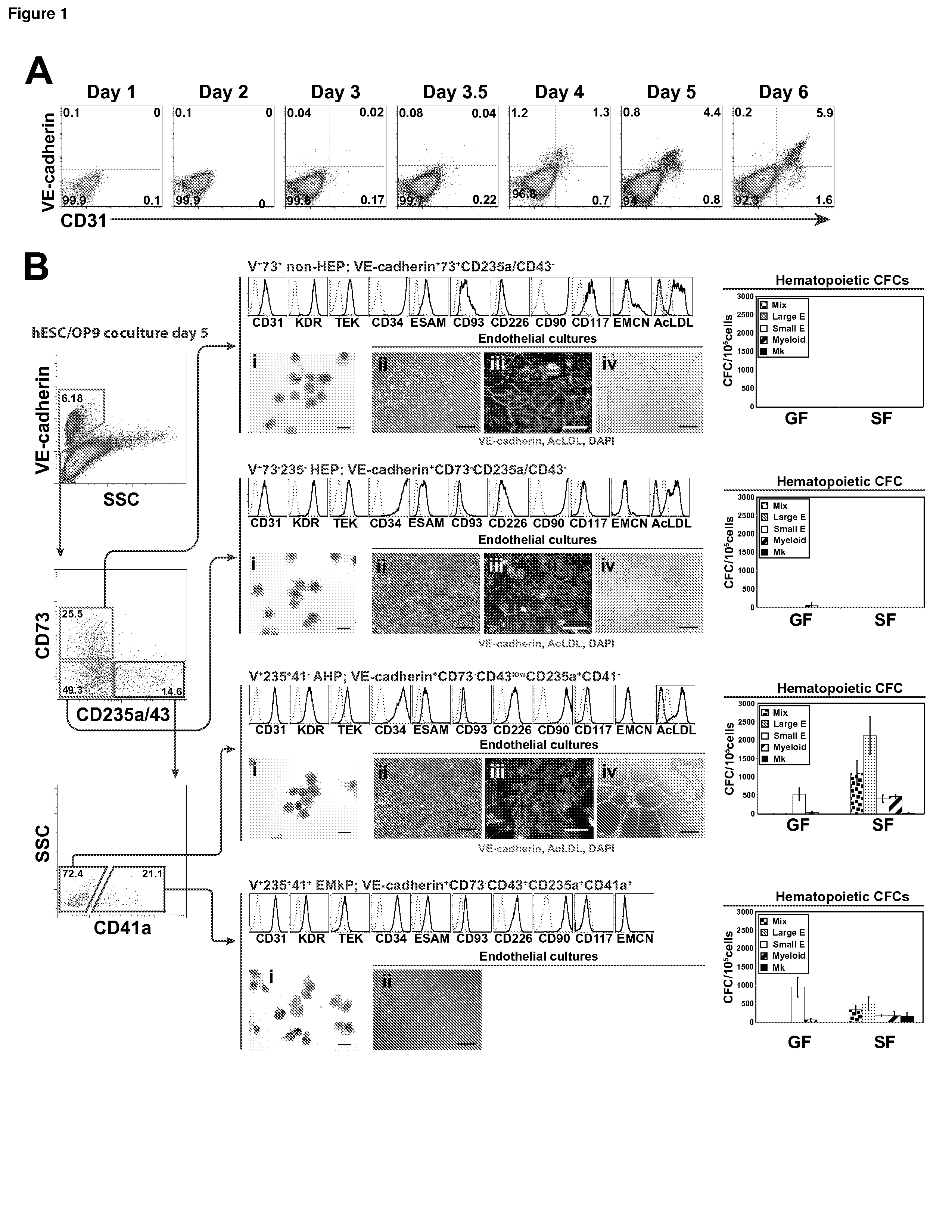
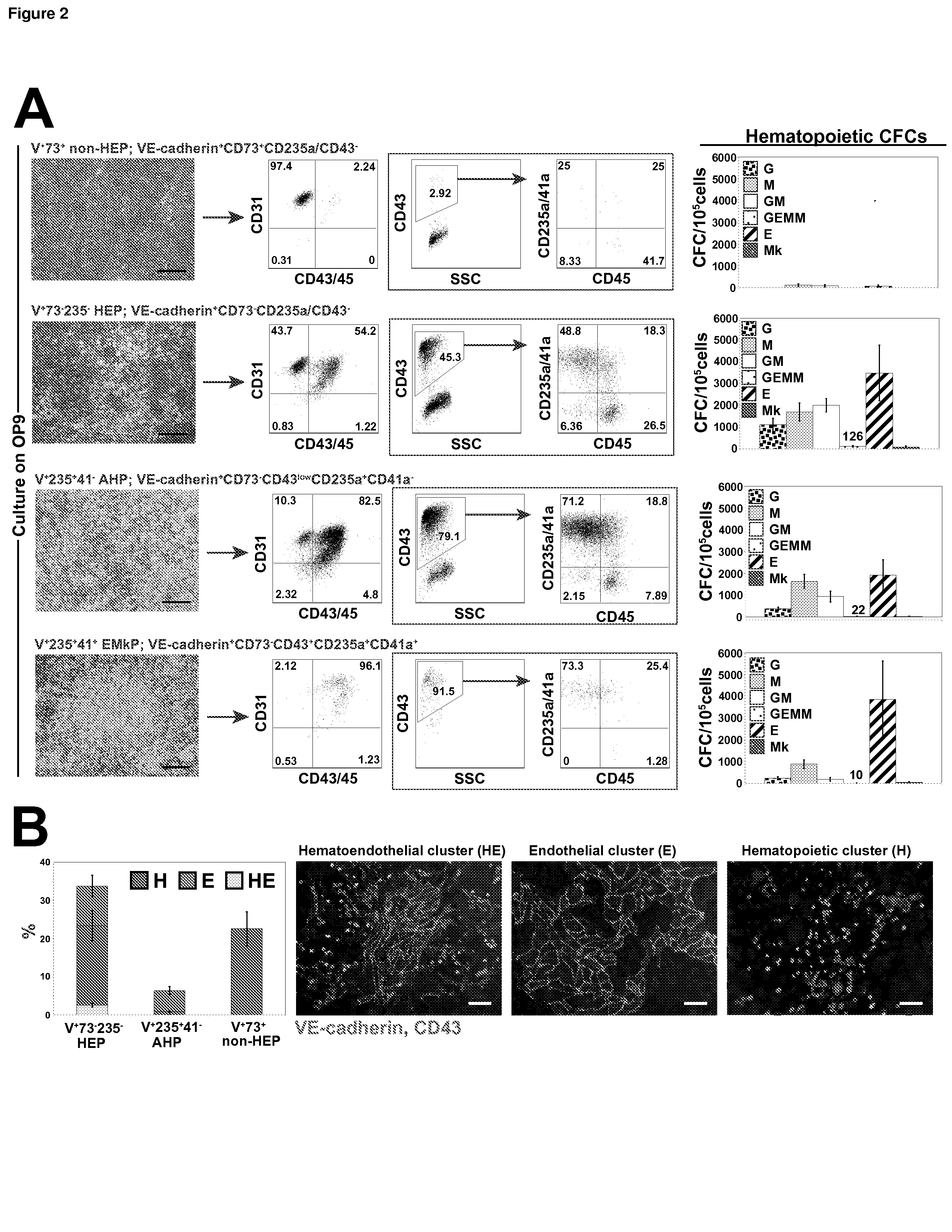
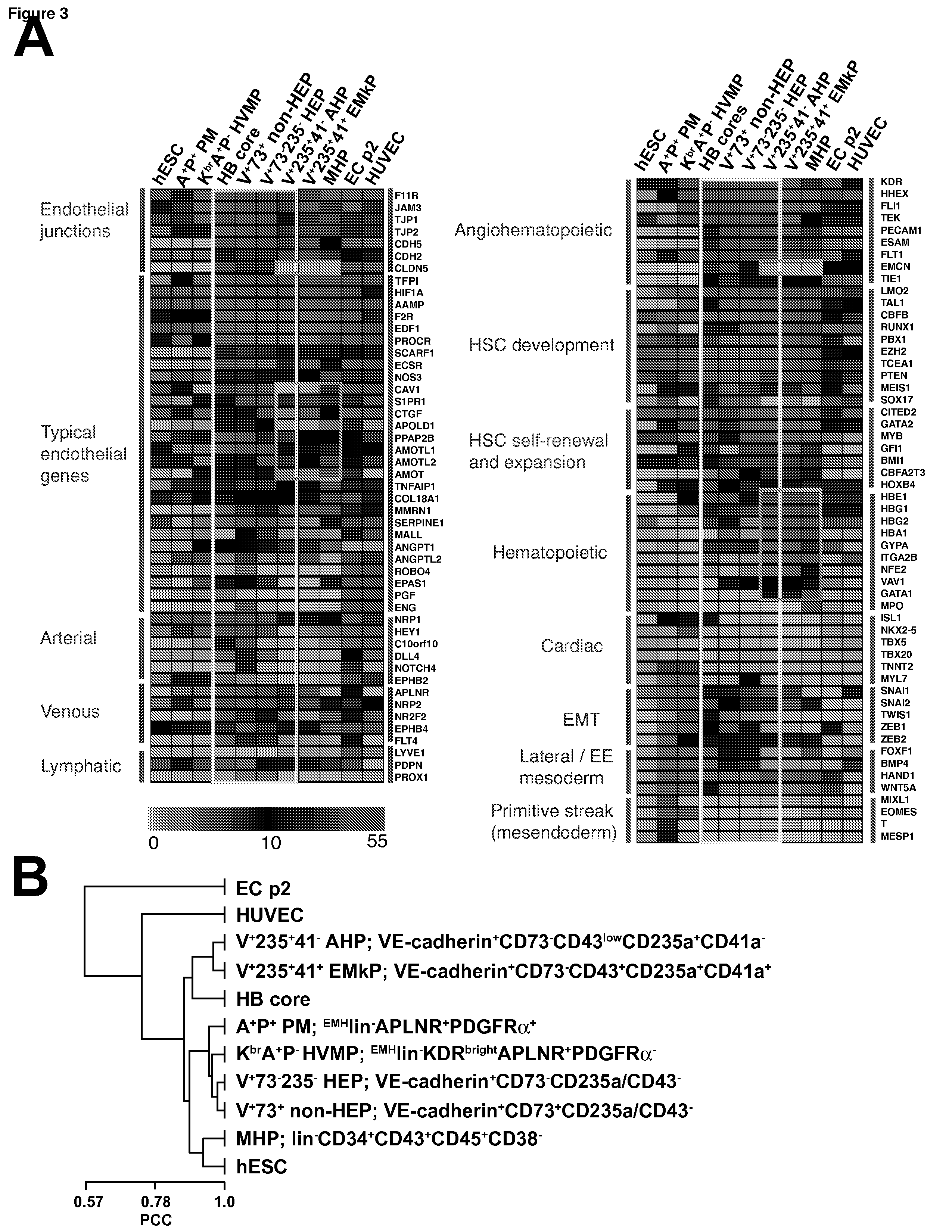
[0069]In the examples below, we show that HE progenitors (HEPs) can be generated from hPSCs and be identified precisely based on VE-cadherin (CD144) expression but the lack of CD73 and CD235a / CD43 expression. We demonstrate that HEPs represent a transient population of cells with the stroma-dependent capacity to generate the entire spectrum of myeloid progenitors including β-hemoglobin producing erythroid cells and pan-myeloid CFC-GEMM. In addition, we found that the earliest VE-cadherin+CD73−CD43lowCD235a+CD41a− blood cells retain endothelial potential and possess a unique FGF2-dependent hematopoietic colony-forming activity. A novel population of endothelial progenitors lacking hematopoietic potential (non-HEPs) was distinctively recognized by the expression of CD73 and a high level of CD117, i.e. VE-cadherin+CD73+CD235a / CD43−CD117high phenotype. VE-cadherin+CD73−CD43 / CD235a−HE cells originated from EMHlin−KDRbrightAPLNR+PDGFRαlow / − hematovascular mesodermal precursors...
PUM
| Property | Measurement | Unit |
|---|---|---|
| densities | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| imaging | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com