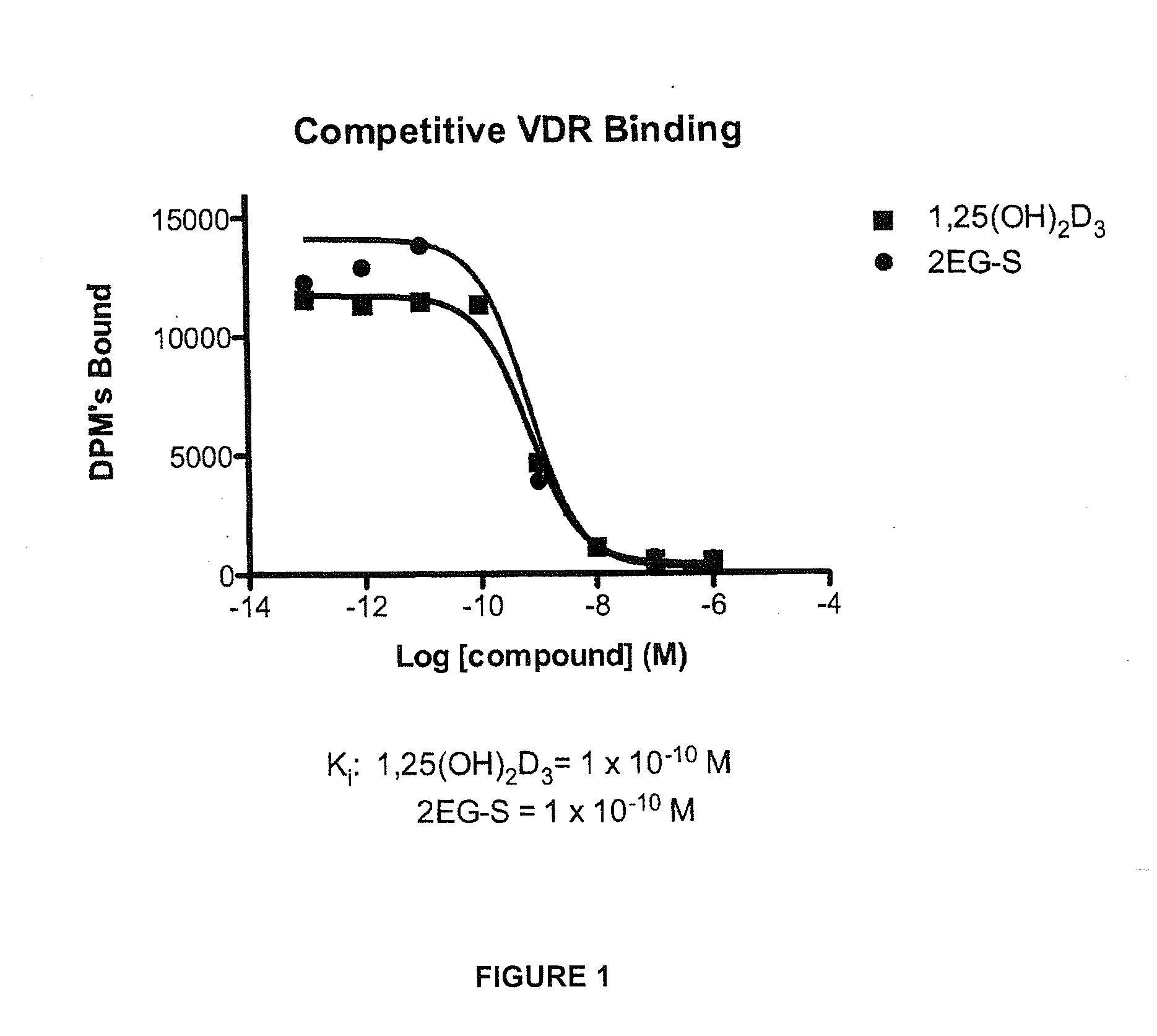
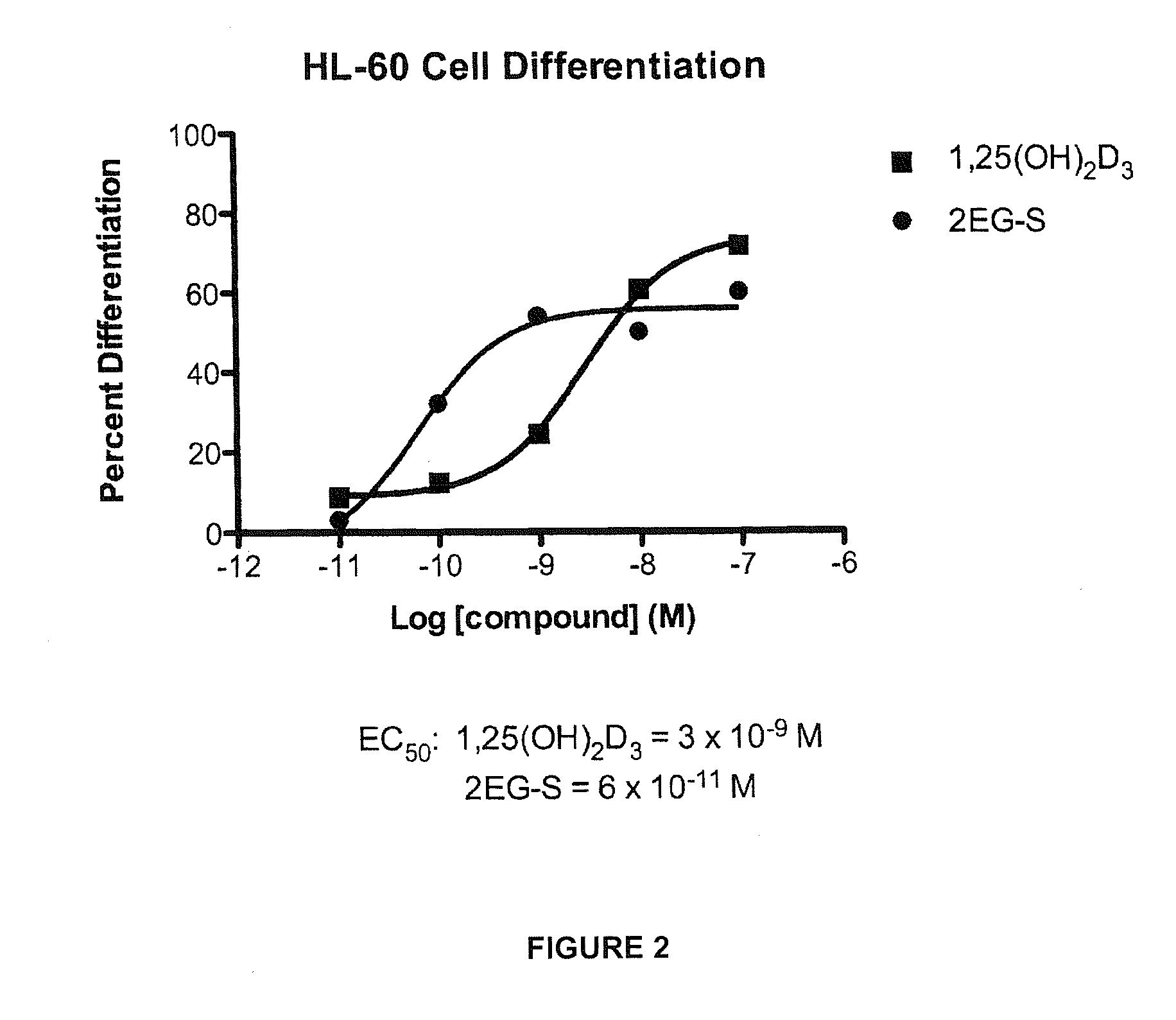
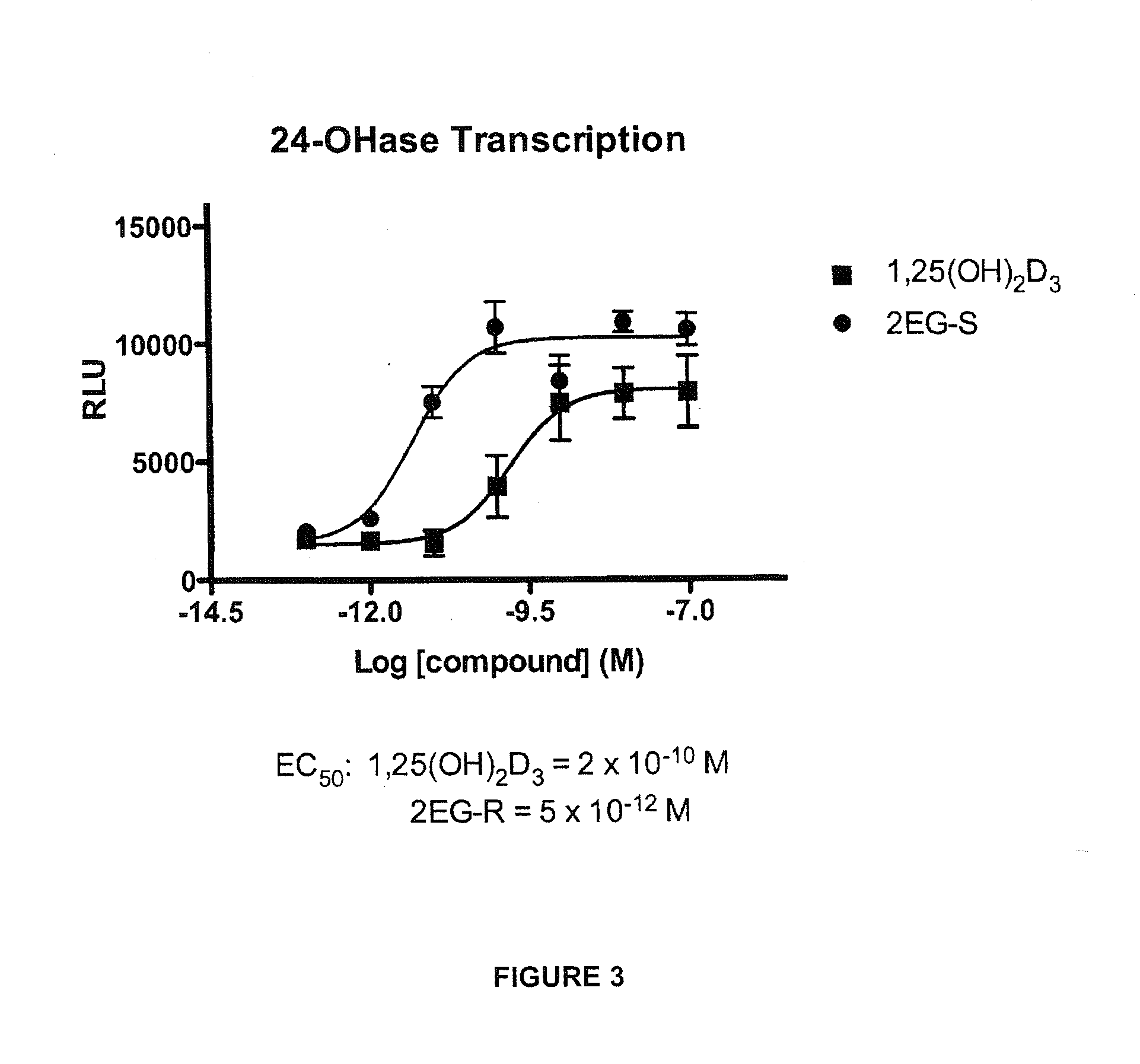
2-methylene-vitamin d analogs and their uses
a technology of vitamin d and analogs, applied in the field of vitamin d compounds, can solve problems such as the flattening of the cyclohexanediol ring, and achieve the effects of high binding to vitamin d receptors, high transcriptional activity, and high activity in their ability to mobilize calcium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0046]Chemistry. Melting points (uncorrected) were determined on a Thomas-Hoover capillary melting-point apparatus. Optical rotations were measured in chloroform using a Perkin-Elmer 241 automatic polarimeter at 22° C. Ultraviolet (UV) absorption spectra were recorded with a Perkin-Elmer Lambda 3B UV-VIS spectrophotometer in ethanol. 1H nuclear magnetic resonance (NMR) spectra were recorded in deuteriochloroform at 400 and 500 MHz with a Bruker DMX-400 and Bruker DMX-500 spectrometers, respectively. 13C nuclear magnetic resonance (NMR) spectra were recorded in deuteriochloroform at 100 and 125 MHz with a Bruker DMX-400 and Bruker DMX-500 spectrometers, respectively. Chemical shifts (δ) were reported downfield from internal Me4Si (δ 0.00). Electron impact (EI) mass spectra were obtained with a Micromass AutoSpec (Beverly, Mass.) instrument. High-performance liquid chromatography (HPLC) was performed on a Waters Associates liquid chromatograph equipped with a Model 6000A solvent deliv...
example i
Preparation of 1α,25-dihydroxy-2-methylene-vitamin D3 (15)
[0048](a) Wittig reaction of the ketone 1 (SCHEME I). (1R,3R,5R)-1-Acetoxy-3-[(tert-butyldimethylsilyl)oxy]-4-methylene-6-oxabicyclo[3.2.1]octan-7-one (2). A solution of potassium tert-butoxide in THF (1.0 M; 746 μL, 746 μmmol) was added dropwise to a stirred suspension of methyl triphenylphosphonium bromide (280 mg, 784.6 μmol) in anhydrous THF (5.5 mL) at 0° C. The mixture was warmed up to room temperature and stirred for additional 10 min. A solution of ketone 1 (126 mg, 382.7 μmol) in THF (1.6 mL) was added via cannula and stirring was continued at room temperature for 1 h. Water was added and the mixture was extracted with ethyl acetate, dried over MgSO4 and concentrated. The residue was applied on a silica Sep-Pak cartridge and eluted with hexane / ethyl acetate (95:5) to afford compound 2 (91 mg, 73%).
[0049]2: [α]20D −79° (c 1.0 CHCl3); 1H NMR (500 MHz, CDCl3) δ 0.086 (6H, s, 2×SiCH3), 0.921 (9H, s, Si-t-Bu), 2.06 (1H, b...
example ii
Preparation of (20S)-1α,25-dihydroxy-2-methylene-vitamin D3 (21) and (5E)-(20S)-1α,25-dihydroxy-2-methylene-vitamin D3 (22)
[0071](a) Conversion of the Grundmann ketone 16 to the enol triflate 17 (SCHEME III). (20S)-25-[(Triethylsilyl)oxy]-8-trifluoromethanesulfonyloxy-des-A,B-cholest-8-ene (17). A solution of the ketone 16 (28.5 mg, 72.19 mmol) in anhydrous THF (350 μL) was slowly added to the solution of LDA (2.0 M in THF / heptane / ethylbenzene; 40 μL, 80 μmol) in dry THF (100 μL) at −78° C. under argon. Then a solution of N-phenyltriflimide (28.3 mg, 79.27 mmol) in dry THF (100 μL) was added. After 2 h cooling bath was removed and reaction mixture was allowed to warm up to room temperature. Stirring was continued for 30 min and water was added. The mixture was extracted with hexane, dried over MgSO4 and concentrated. The residue was applied on a silica Sep-Pak cartridge and eluted with hexane to afford the enol triflate 17 (17.2 mg, 82% considering recovered substrate) and unreacted...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com