Methods of enhancing antibody-dependent cellular cytotoxicity
a cytotoxicity and antibody technology, applied in the field of enhancing the cytotoxicity of therapeutic monoclonal antibodies, can solve the problems of low antibody activity, increased dosage and cost of treatment, and insufficient therapeutic effects on cancer, so as to increase the efficiency of a therapeutic monoclonal antibody and enhance the therapeutic effect of monoclonal antibodies. , the effect of increasing the efficiency of a therapeutic monoclonal antibody and increasing the ad
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Enhancement of the Therapeutic Effectiveness of Monoclonal Antibody Therapy by Enhancing NK Activity and ADCC Including ADCC in Genetically Resistant Populations
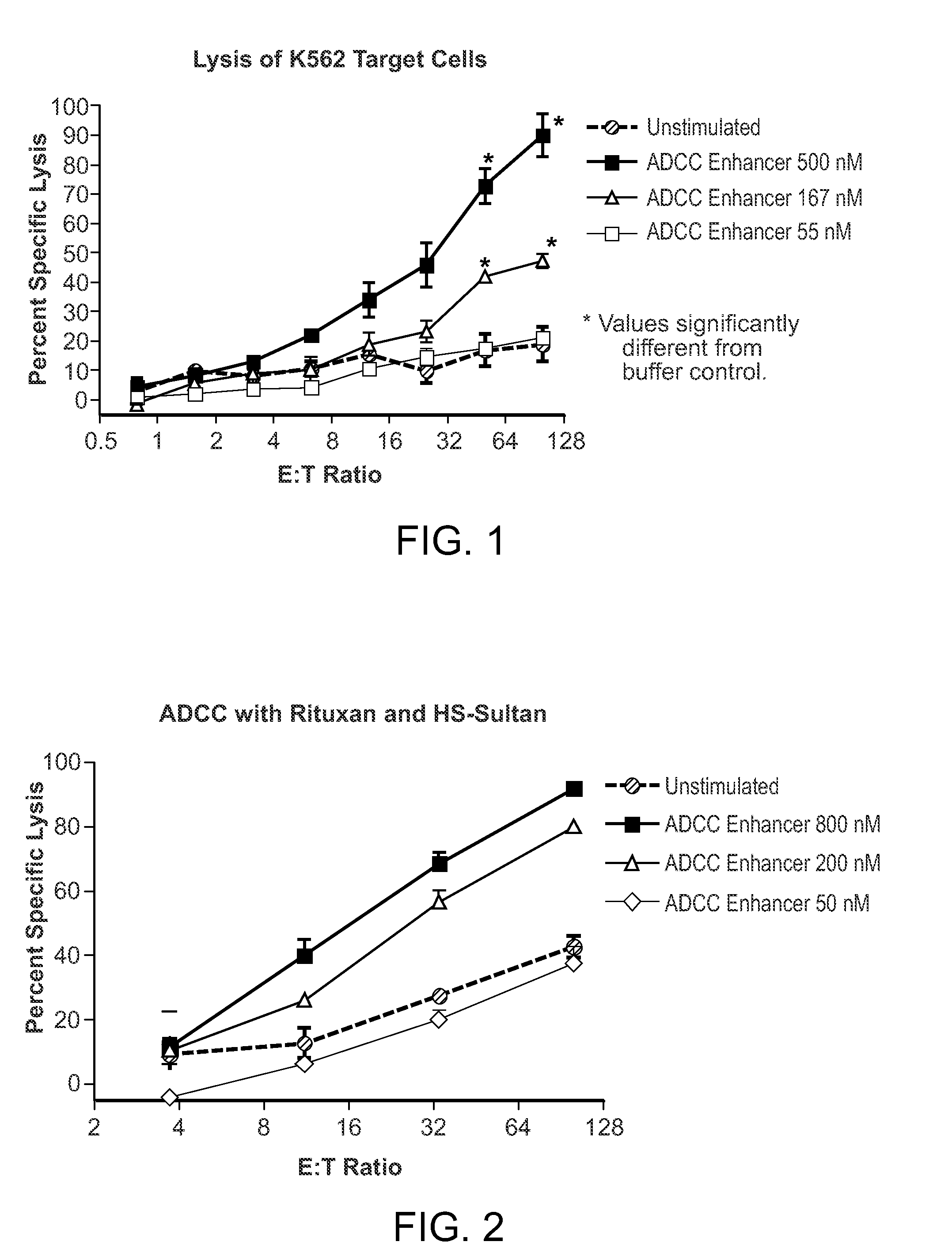
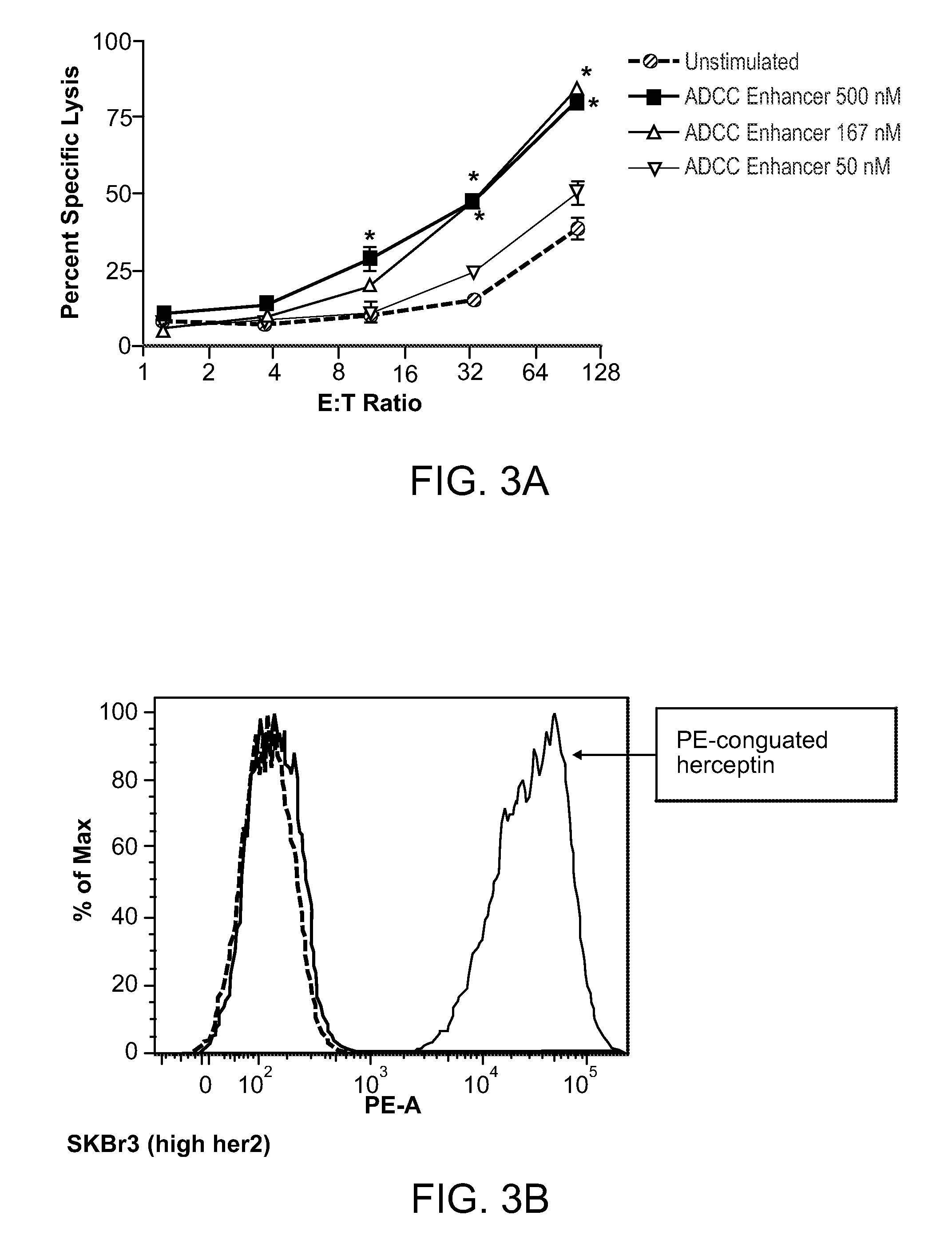
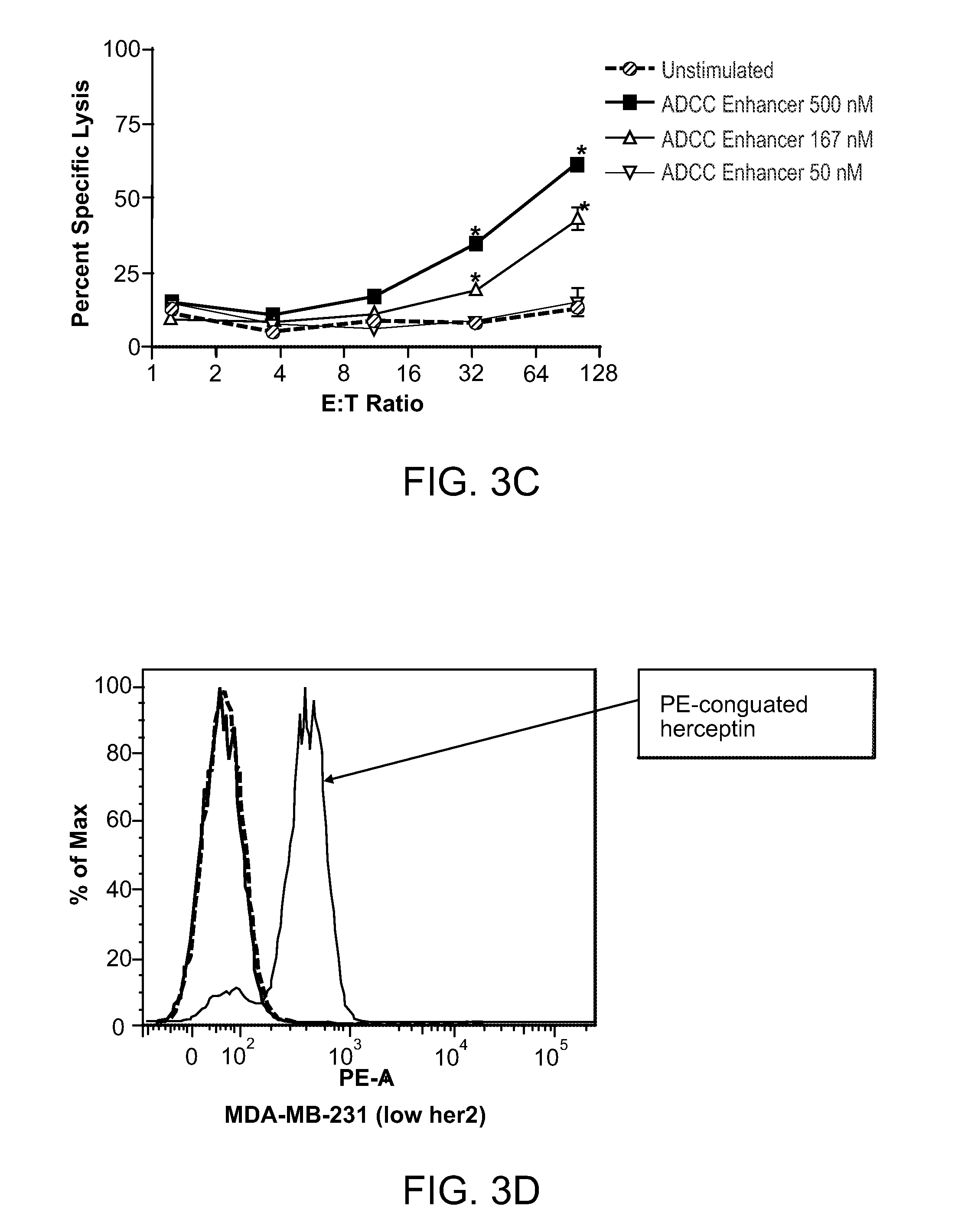
[0190]A subcutaneous formulation comprising the ADCC enhancer {2-amino-8-[4-(pyrrolidinylcarbonyl)phenyl]-(3H-benzo[f]azepin-4-yl)}-N,N-dipropylcarboxamide was used for the experiments. The structure for {2-amino-8-[4-(pyrrolidinylcarbonyl)phenyl]-(3H-benzo[f]azepin-4-yl)}-N,N-dipropylcarboxamide is as follows:
[0191]Stimulation of PBMCs:
[0192]Human Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll™ density gradient centrifugation and resuspended in RPMI containing 2% heat-inactivated FBS. PBMCs at a concentration of 1 to 3 million cells per mL were incubated with 10-500 nM ADCC enhancer in a humidified CO2 incubator for 18-72 hrs. Activated PBMCs were then used as effector cells in NK and ADCC assays.
[0193]NK and ADCC Assays:
[0194]PBMCs activated with the ADCC enhancer were tested for the ability to enhance ...
example 2
Polymorphisms of FcγR and Cancer Treatment
[0199]ADCC is mediated through immune effector cells included NK cells that engage the Fc portion of the monoclonal antibody through specific receptors. Patients with single nucleotide polymorphisms in these receptors such as FcRgamma3a position 158 and FcRgamma2a position 131 have a poorer clinical prognosis presumably from poor ADCC due to a lower affinity of the receptor for the monoclonal antibody. Previous studies have found that a polymorphism in the FcγR3A molecule (158F / V) that alters the molecule's affinity for IgG1 is an important factor determining the clinical efficacy seen with some monoclonal antibodies (mAbs) used in the treatment of cancer. To determine if this common polymorphism affects the baseline antibody-dependent cellular cytoxicity (ADCC) response and / or response to the ADCC enhancer molecule of the present invention (Compound VTX-2337), PBMCs from donors were genotyped for the two alleles encoding the F and V isoform...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com