Apparatus and methods for optical stimulation of neural tissues
a neural tissue and optical stimulation technology, applied in the field of neural tissue stimulation, can solve the problems of poor spatial specificity, coagulation or ablation of the target tissue, easy electrical interference of the electrical stimulation, etc., and achieve the effect of minimal tissue damag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Biophysical Mechanism Responsible for Low-Level, Transient Optical Stimulation of Peripheral Nerve
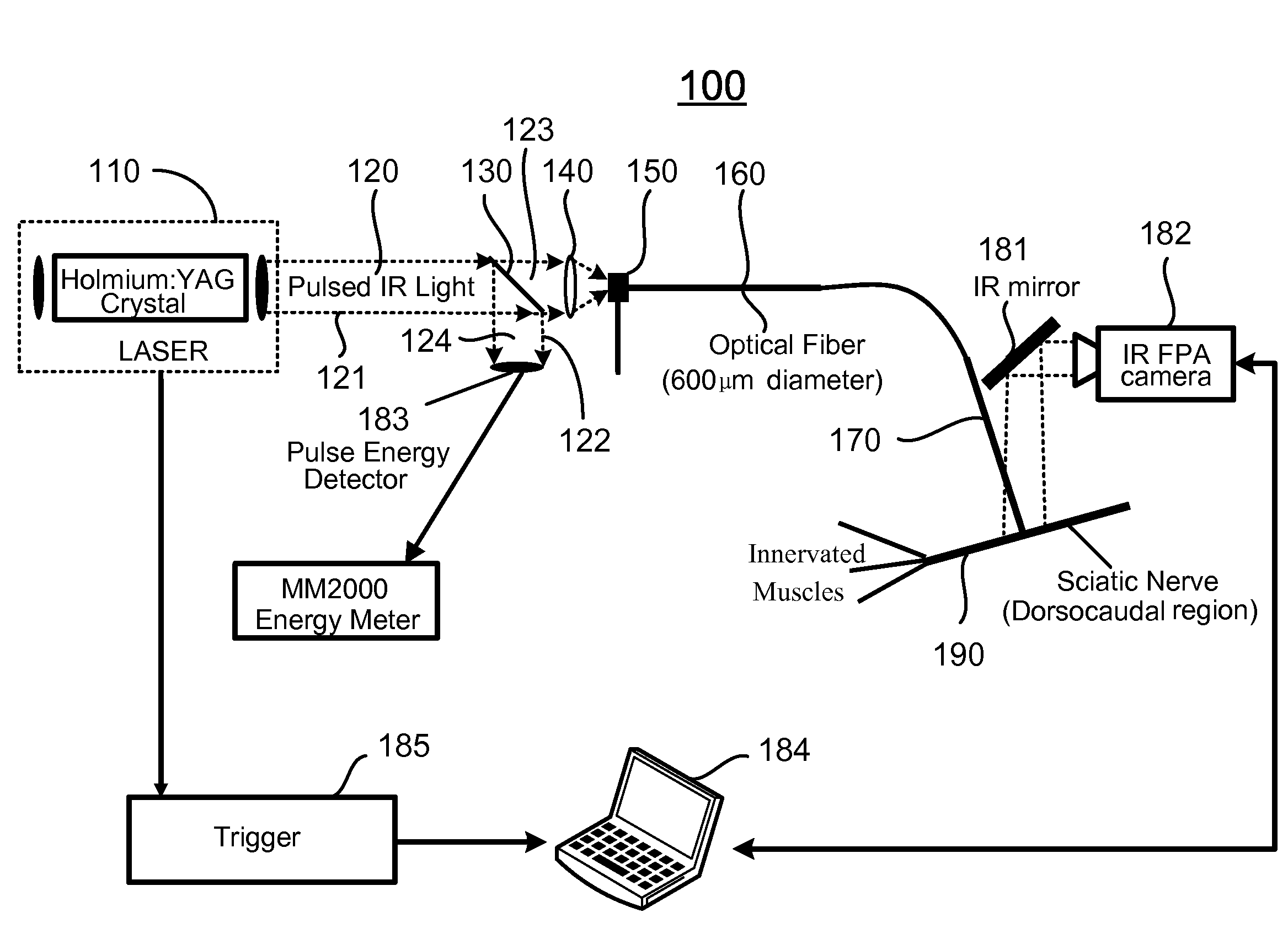
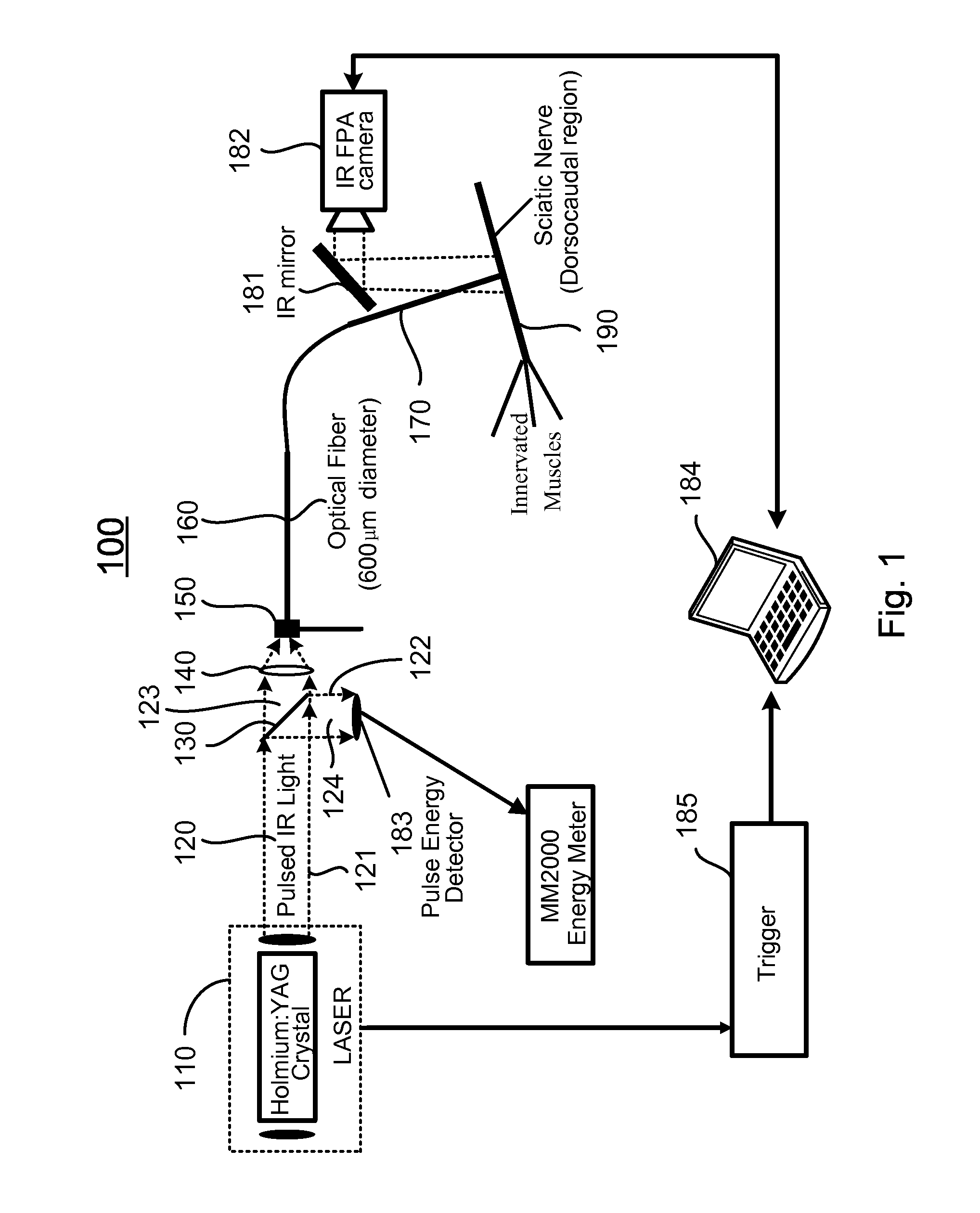
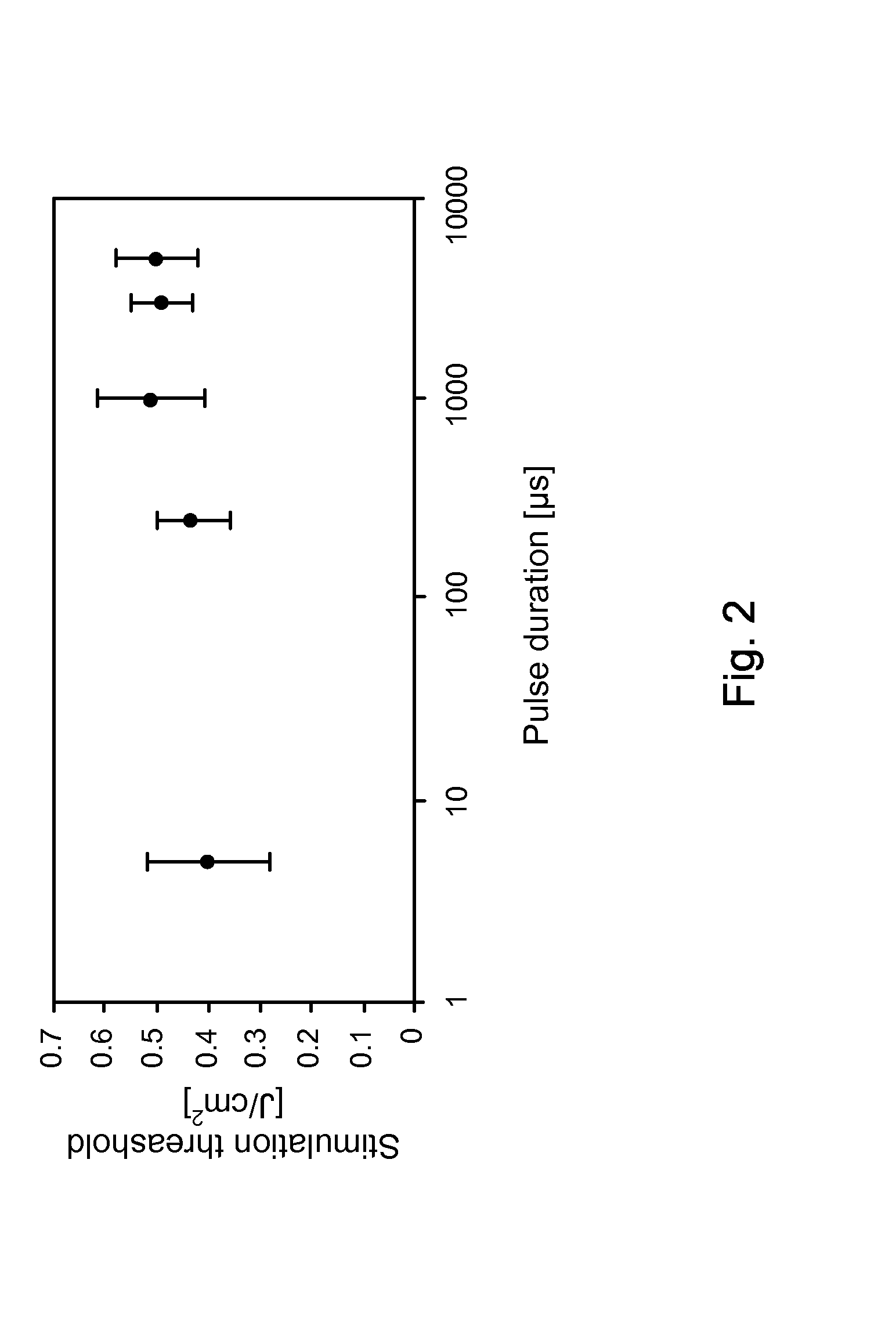
[0079]In vivo neural activation with low-levels of pulsed infrared light [1] exhibits advantages over standard electrical means by providing a contact-free, spatially selective, artifact-free stimulation method [2] that encourages development towards clinical application. In this example, the biophysical mechanism underlying this phenomenon was determined by careful examination of possible photobiological effects following absorption driven light-tissue interaction. Sciatic nerve preparation was stimulated in vivo with the Holmium:YAG laser (2.12 μm), Free Electron Laser (2.1 μm), Alexandrite laser (690 nm), and the commercial prototype solid state laser nerve stimulator built by Aculight (1.87 μm), respectively. Through a process of elimination approach, relative contributions to the neural activation were systematically determined from interaction types resulting in optical stimulatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com