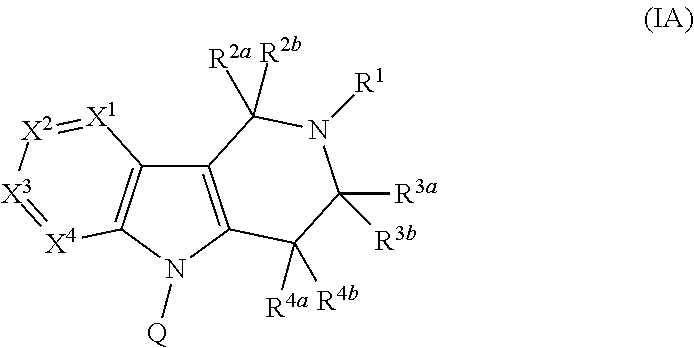
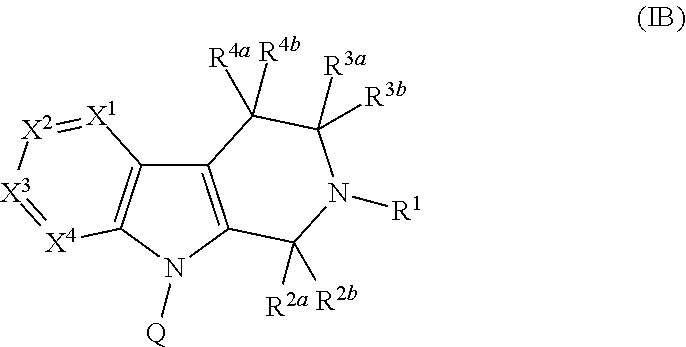
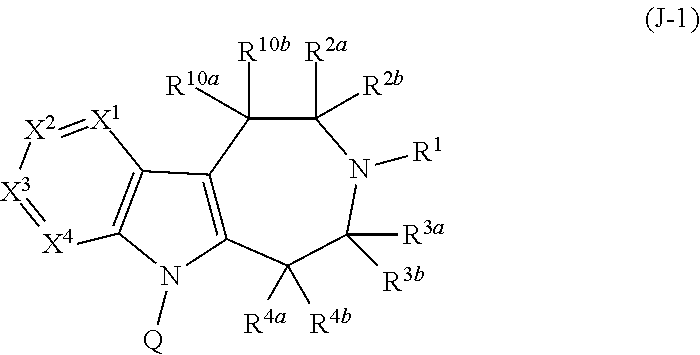
Compounds and methods of treating diabetes
a technology of compound and diabetes, applied in the field of compound and diabetes treatment, to achieve the effects of promoting insulin release in the blood stream, reducing blood glucose levels, and increasing insulin secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example no.1
Example No. 1
Preparation of Compound No. 1
[0441]A solution of 2,8-dimethyl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole (0.2 g, 1 mmol), bromobenzene (0.314 g, 2 mmol), K3PO4 (0.424 g, 2 mmol), CuI (19 mg, 0.1 mmol) and L-Proline (23 mg, 0.2 mmol) in dry DMF (3 mL) was stirred at 150° C. for 12 h. The reaction mixture was diluted with water and extracted with EtOAc. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford crude material, which was purified by reverse phase HPLC to yield 2,8-dimethyl-5-phenyl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole as an off white solid (83 mg). 1H NMR(HCl salt, CD3OD) δ (ppm): 7.60 (t, 2H), 7.5 (t, 1H), 7.4 (d, 2H), 7.35 (s, 1H), 7.1 (d, 1H), 7.05 (d, 1H), 4.8 (d, 1H), 4.4 (d, 1H), 3.85-3.8 (m, 1H), 3.6-3.59 (m, 1H), 3.2-3.19 (m, 1H), 3.18 (s, 3H), 3-2.95 (m, 1H), 2.4 (s, 3H).
example no.2
Example No. 2
Preparation of Compound No. 2
[0442]A solution of 2,8-dimethyl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole (0.2 g, μmol), 4-bromopyridine (0.316 g, 2 mmol), K3PO4 (0.424 g, 2 mmol), CuI (19 mg, 0.1 mmol) and L-Proline (23 mg, 0.2 mmol) in dry DMF (3 mL) was stirred at 150° C. for 12 h. The reaction mixture was diluted with water and extracted with EtOAc. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford crude material, which was purified by reverse phase HPLC to yield 2,8-dimethyl-5-pyridin-4-yl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole as an off white solid (30 mg). 1H NMR(HCl salt, CD3OD) δ (ppm): 8.95 (d, 2H), 8.21 (d, 2H), 7.65 (d, 1H), 7.21 (s, 1H), 7.21 (d, 1H), 4.8 (d, 1H), 4.4 (d, 1H), 3.95-3.9 (m, 1H), 3.6-3.50 (m, 2H), 3.25 (m, 1H), 3.2 (s, 3H), 2.5 (s, 3H).
example no.3
Example No. 3
Preparation of Compound No. 3
[0443]A solution of 2,8-dimethyl-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole (0.2 g, 1 mmol), 5-bromo-2-methyl-pyridine (0.348 g, 2 mmol), K3PO4 (0.424 g, 2 mmol), CuI (19 mg, 0.1 mmol) and L-Proline (23 mg, 0.2 mmol) in dry DMF (3 mL) was stirred at 150° C. for 12 h. The reaction mixture was diluted with water and extracted with EtOAc. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford crude material, which was purified by reverse phase HPLC to yield 2,8-dimethyl-5-(6-methyl-pyridin-3-yl)-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole as semisolid (8.6 mg). 1H NMR(HCl salt, CD3OD) δ (ppm): 8.6 (s, 1H), 8.05 (d, 1H), 7.7 (d, 1H), 7.19 (s, 1H), 7.19-7.05 (dd, 2H), 4.8 (m, 1H), 4.4 (m, 1H), 3.90-3.8 (m, 1H), 3.6-3.50 (m, 1H), 3.25-3.20 (m, 1H), 3.2 (s, 3H), 3.05-3.0 (m, 1H), 2.75 (s, 3H), 2.45 (s, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
| Level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com