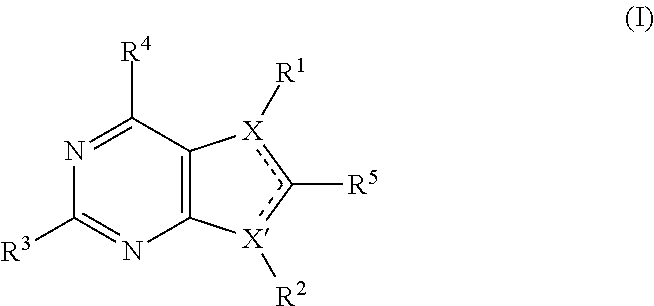
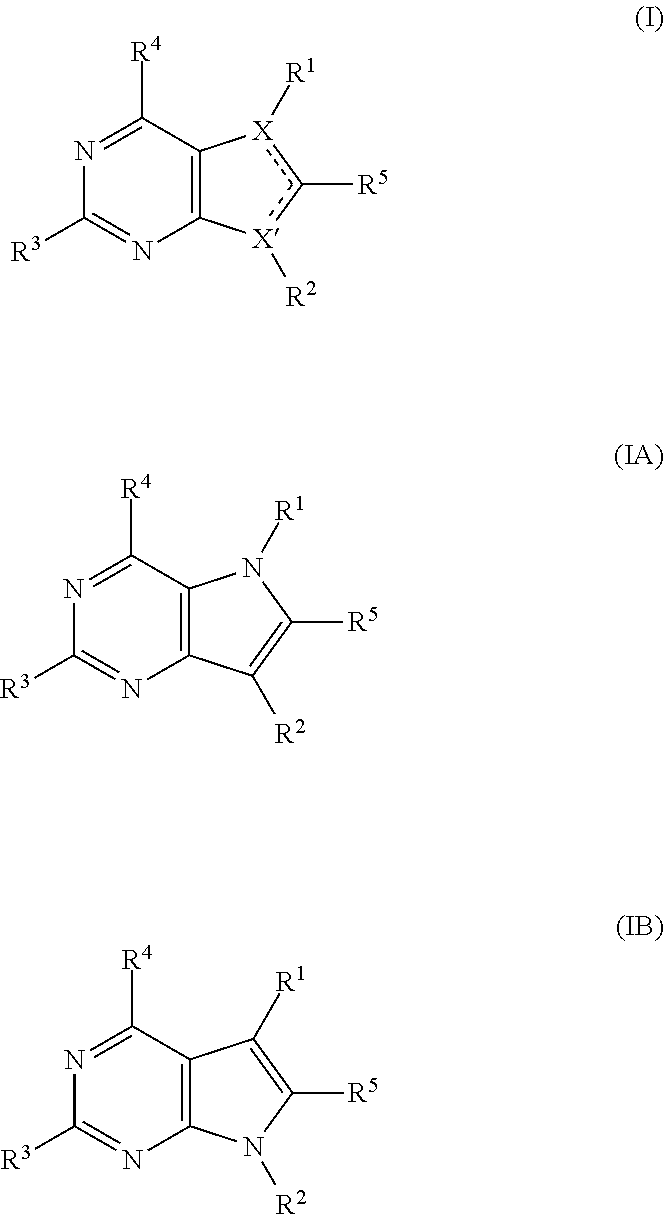
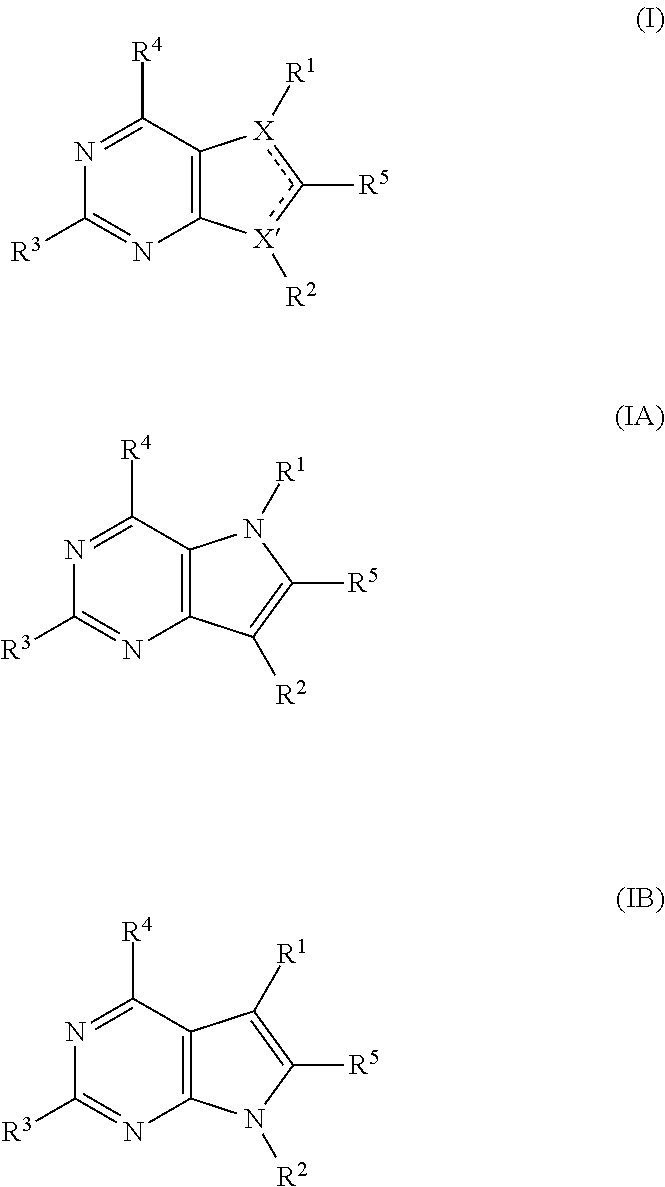
Pyrrolopyrimidine compounds for the treatment of cancer
a technology of pyrrolopyrimidine and compounds, applied in the field of compounds, can solve the problems of late complications, resistance to and relapse, and pediatric cancer death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
7-((Trans-4-aminocyclohexyl)methyl)-N-butyl-5-(4-fluorophenyl)-7H-pyrrolo[2,3-d]pyrimidin-2-amine
General Procedure A:
[0098]
tert-Butyl trans-4-((5-bromo-2-(butylamino)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)methyl)cyclohexylcarbamate
[0099]
[0100]A 10 mL microwave tube was charged with 5-bromo-2-chloro-7H-pyrrolo[2,3-d]pyrimidine (0.23 g, 1.0 mmol), tert-butyl trans-4-(iodomethyl)cyclohexylcarbamate (0.51 g, 1.5 mmol), K2CO3 (0.28 g, 2.0 mmol), DMSO (1.5 mL) and THF (3 mL). The mixture was heated at 150° C. for 100 min in microwave. After the reaction mixture was cooled to ambient temperature, n-butylamine (0.18 g, 2.5 mmol) was added. The mixture was heated at 150° C. for 90 min in microwave. After cooling to ambient temperature, the reaction was poured into water and extracted with EtOAc (3×). The combined organic layer was dried (Na2SO4) and concentrated. The crude mixture was purified by Isco to provide tert-butyl trans-4-((5-bromo-2-(butylamino)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)methyl)cyc...
example 2
Trans-4-(2-(Butylamino)-5-(4-fluorophenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)cyclohexanol
General Procedure B:
[0103]
5-Bromo-7-(trans-4-((tert-butyldimethylsilyl)oxy)cyclohexyl)-2-chloro-7H-pyrrolo[2,3-d]pyrimidine
[0104]
[0105]To a suspension of 5-bromo-2-chloro-7H-pyrrolo[2,3-d]pyrimidine (0.13 g, 0.50 mmol) and cis-4-(tert-butyldimethylsilyloxy)cyclohexanol (0.23 g, 1.0 mmol) in toluene (8 mL) was added (cyanomethylene)trimethylphosphorane (CMMP; prepared according to Chem. Pharm. Bull. 2003, 51(4), 474-476.) (6.3 mL, 0.16 M in THF, 1.0 mmol). The resulting clear solution was refluxed for 16 h. The reaction mixture was washed with brine, and extracted with EtOAc (3×). The combined organic layer was dried (Na2SO4) and concentrated. The residue was purified on ISCO to provide the desired product (0.16 g, 72%). 1H NMR (400 MHz, CD3OD) δ 8.71 (s, 1H), 7.27 (s, 1H), 4.70 (tt, J=12.2, 3.9 Hz, 1H), 3.69 (tt, J=10.5, 4.2 Hz, 1H), 2.09-1.99 (m, 3H), 1.86-1.71 (m, 2H), 1.66-1.54 (m, 3H), 0.90 (s...
example 3
Cis- and Trans-(1r,4r)-4-(2-(butylamino)-5-(4-fluorophenyl)-5H-pyrrolo[3,2-d]pyrimidin-7-yl)cyclohexanol
General Procedure C:
[0109]
N-Butyl-5H-pyrrolo[3,2-d]pyrimidin-2-amine
[0110]
[0111]A suspension of 2-chloro-5H-pyrrolo[3,2-d]pyrimidine (0.62 g, 4 mmol) in 5 mL iPrOH was added nBuNH2 (2.5 mL, 25.3 mmol) and followed by HCl (2.0 mL, 4.0 M in dioxanes, 8 mmol). The resulting solution was heated at 170° C. for 1 h under microwave irradiation. The reaction was monitored by LC-MS. The reaction time should be extended whenever it is necessary. After evaporation of solvents, the crude product was washed with minimal amount of MeOH. The solid was collected. And the MeOH filtrate was purified by ISCO to provide the desired product (0.73 g, 96%). 1H NMR (400 MHz, CD3OD) δ 8.42 (d, J=0.8 Hz, 1H), 7.53 (d, J=3.1 Hz, 1H), 6.27 (dd, J=3.0, 0.8 Hz, 1H), 3.37 (t, J=7.1 Hz, 2H), 1.68-1.57 (m, 2H), 1.52-1.36 (m, 2H), 0.97 (t, J=7.4 Hz, 3H); MS m / z 191.2 [M+H]+.
N-Butyl-5-(4-fluorophenyl)-5H-pyrrolo[3,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Structure | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
| Covalent bond | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap