Rupture-resistant compliant radiopaque catheter balloon and methods for use of same in an intravascular surgical procedure
a radiopaque catheter and catheter shaft technology, applied in the field of medical devices, can solve the problems of warping of the catheter shaft, limiting the usefulness or marketability of the catheter, harming the patient, etc., and achieve the effect of marked increase in the deflation time before the removal of the balloon from the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fabrication of Compliant Radiopaque Balloon Having a Braided Fiber Layer
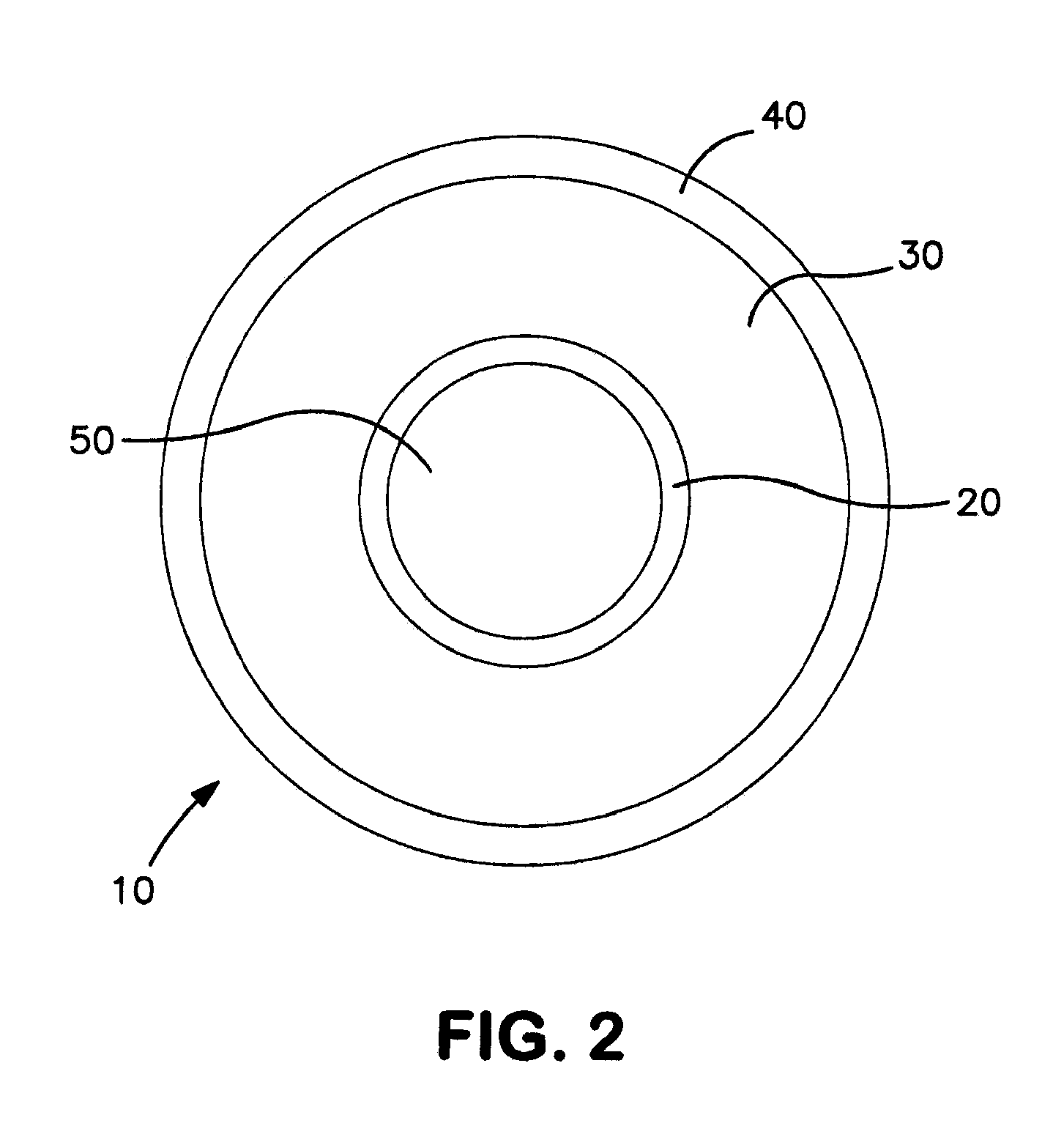
[0069]Balloons were constructed having the general cross-sectional design configuration depicted in FIG. 2. Shown in an inflated state, the balloons generally included an inflation layer 20, a fiber layer 30, and a coating layer 40. The inflation layer 20 defines a lumen 50 for retention of inflation fluid used to increase the internal pressure of the lumen 50 to inflate the balloon 10. With reference to FIGS. 2 and 4, the balloons included the following components: inner balloon or inflation layer 20, fiber layer 30, coating layer 40, and deposited directly on the outer surface of the inflation layer 200 is radiopaque layer 210. Materials used for each component are shown in Table 1 as follows.
TABLE 1Balloon Component Materials ListBalloon ComponentMaterial Description / SpecificationInflation LayerVestamid ® Nylon(Inner Balloon) (20)RadiopaqueEpoxy based ink with >95% tungstenCoating (210)Fiber Layer (30)Ultra H...
example 2
Fabrication of Compliant Radiopaque Balloon Having a Fiber Layer Including Braided and Non-Braided Fiber
[0073]Balloons were constructed in a process similar to that discussed in Example 1 with variations to the fiber layer 30. For example, a balloon was constructed including in which the fiber layer 30 includes both a first non-braided layer and a second braided fiber layer. The balloon materials are those shown in Table 1.
[0074]To construct the balloons, inflation layer 20 was first formed from compliant nylon material using a blow molding process. Next, radiopaque coating 210 was optionally applied to the outer surface of the inflation layer 200 via printing in a longitudinally striped pattern along the ‘working’ length of the balloon (e.g., the region between the conical regions of the balloon) or to substantially the entire outer surface of the inflation layer 200. Fiber layer 30 was next formed by applying a thin coat of adhesive to the outer surface of the inflation layer 200 ...
example 3
Inflation and Deflation Rates of Balloons Utilizing Various Mixtures of Inflation Fluid
[0078]Inflation and deflation rates were tested for a balloon utilizing various ratios of saline to contrast media as inflation fluid. To perform the experiment, a 6 mm diameter by 10 cm balloon (Bard Dorado®) was tested. It is important to note that unlike the balloon of the present invention, the balloon used to perform the experiment requires the inflation fluid to include 50% or greater of contrast media in a surgical setting to be functional for the surgical procedure. Three trails were performed using saline alone and a 50:50 saline to contrast media mixture. The results are shown in Table 2 below.
TABLE 2Balloon Catheter Inflation and Deflation RatesBalloonCatheterSaline50 / 50 Contrast SalineTrialsInflate (Sec.)Deflate (Sec.)Inflate (Sec.)Deflate (Sec.)1 18*8131721591219314101220Avg. 14.509.0012.3318.67
[0079]As shown in Table 2, the deflation times observed show that increasing the viscosity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com