Protein Refolding Method
a refolding method and protein technology, applied in the field of refolding methods, can solve the problems of protein loss, destabilization, and difficulty in refolding techniques for people without experience, and achieve the effects of preventing association and/or aggregation, and facilitating and effectively refolding a protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
[0115]As a protein, human interleukin-6 (rhIL-6: Japanese Patent No. 3200850) prepared from a recombinant E. coli strain was used. The rhIL-6 is insoluble in water (solubility to 100 g of water at 25° C.: 0.001 g or less), and is in a granular form. Aliquots of the insoluble granules containing 4 mg of rhIL-6 were each put into an Eppendorf tube (made of polypropylene: available from Eppendorf Co., Ltd.). The amount of rhIL-6 in the insoluble granules was quantified in advance by the reverse-phase HPLC method described in a published report (Japanese Patent No. 3200850).
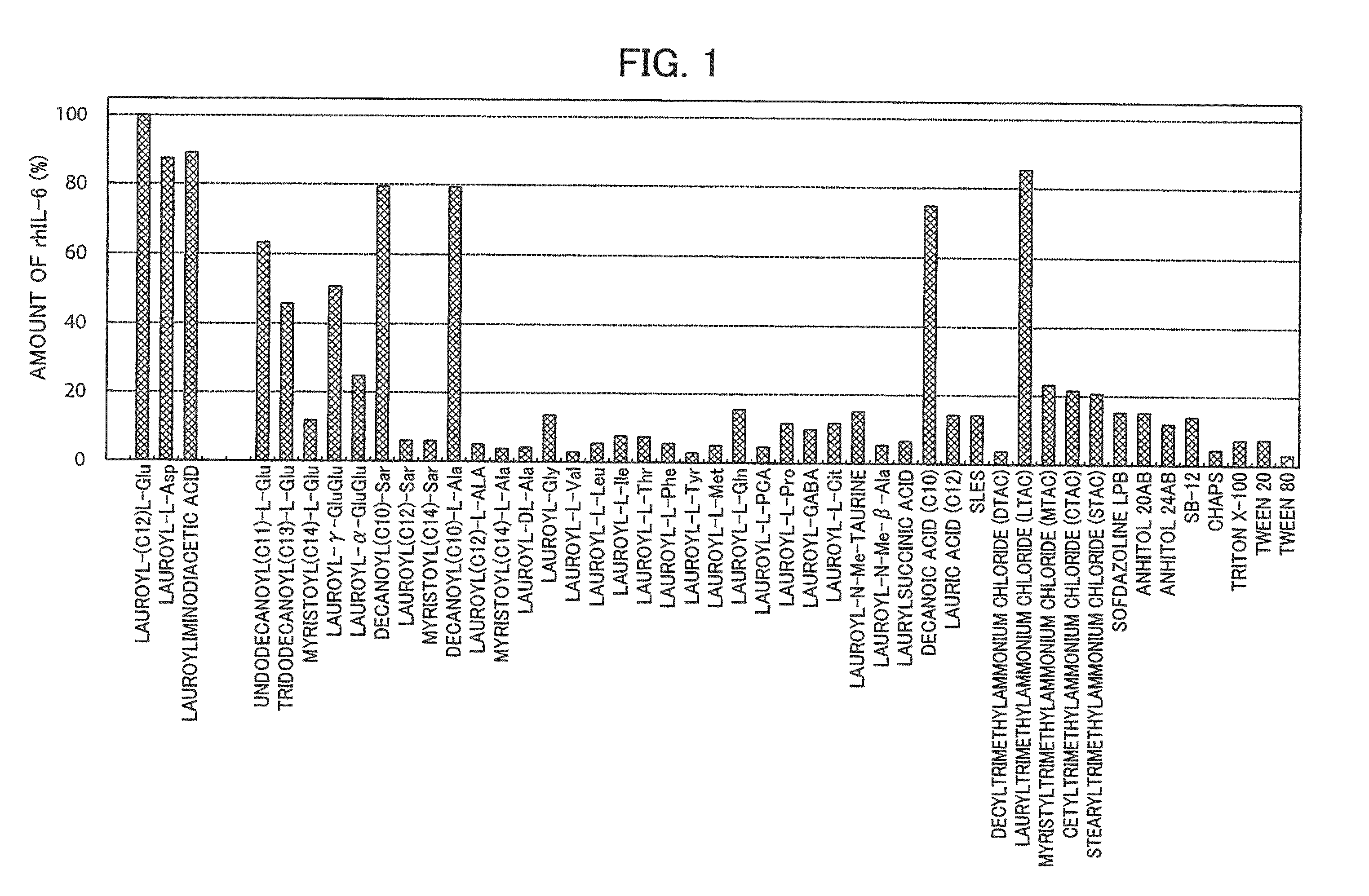
[0116]To each of these, a surfactant prepared in advance to be 5% in pure water (Milli Q water) were appropriately added to achieve a final concentration of lauroyl-L-Glu, lauroyl-L-Asp, lauroyliminodiacetic acid, decanoylSar, decanoyl-L-Ala, decanoic acid, or lauryltrimethylammonium chloride of 2% and the rhIL-6 extraction concentration of 4 mg / ml, and the final volume of 1 ml was adjusted to 1 ml. The mixture was i...
reference example 2
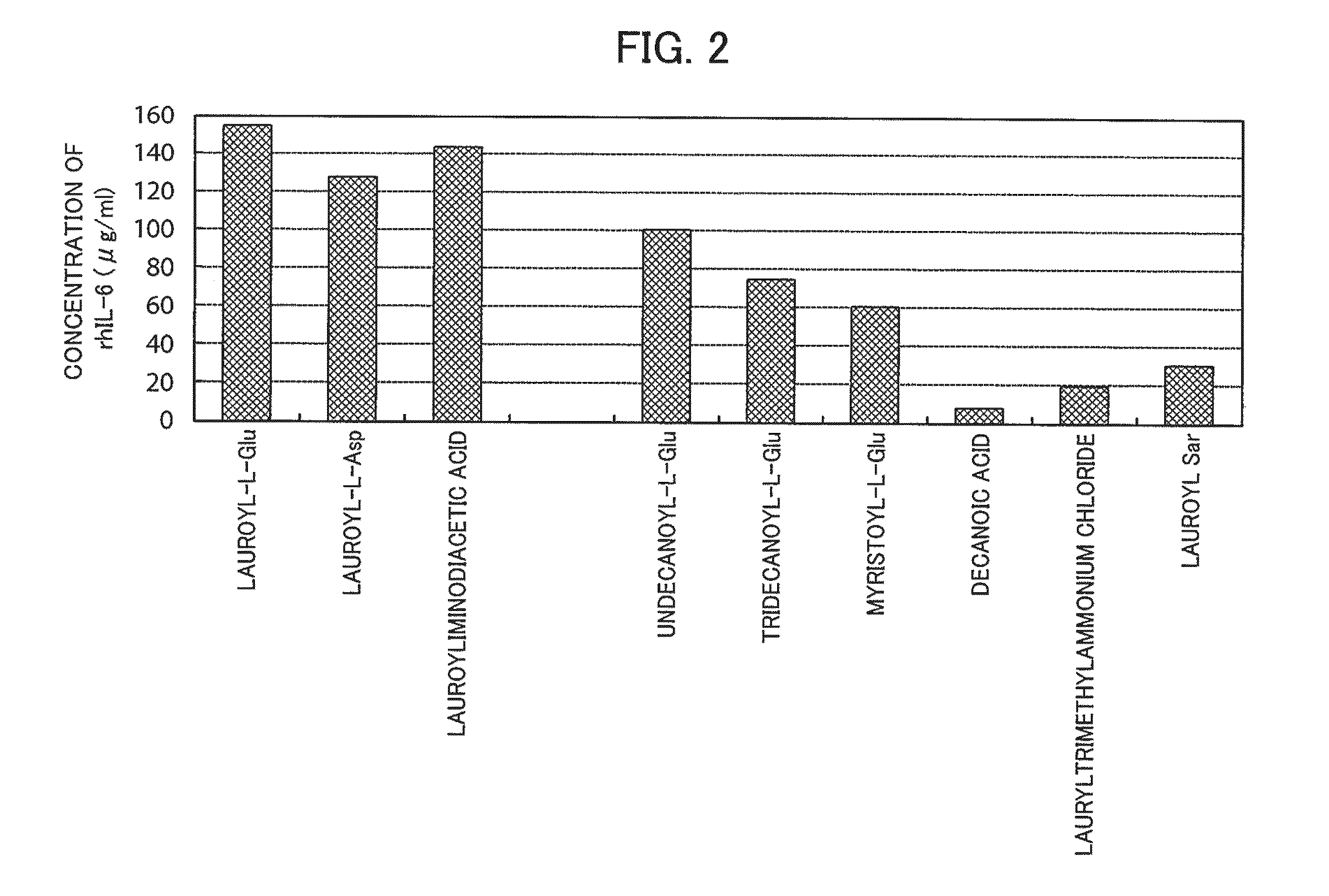
[0124]It was investigated whether lauroyl-L-Glu, lauroyl-L-Asp, and lauroyliminodiacetic acid were effective in restoration of the native higher-order structure of the insoluble rhIL-6, even if the concentration of rhIL-6 to be subjected to extraction from the insoluble granules and solubilization thereafter was increased. For comparison, the following surfactants were used: lauryltrimethylammonium chloride, which resulted in an amount of rhIL-6 of 80% or more as compared to that of lauroyl-L-Glu in Reference Example 1; lauroyl-Sar, which has the same lauroyl group; undecanoyl-L-Glu, tridecanoyl-L-Glu, and myristoyl-L-Glu, which were used for confirmation of the effect of the length of the acyl chain in lauroyl-L-Glu; and decanoic acid, which was used for confirmation of the effect of the acyl chain.
[0125]Aliquots of 13 mg of the same insoluble granules of rhIL-6 as used in Reference Example 1 were each put into an Eppendorf tube. The surfactants were added to the respective Eppendo...
reference example 3
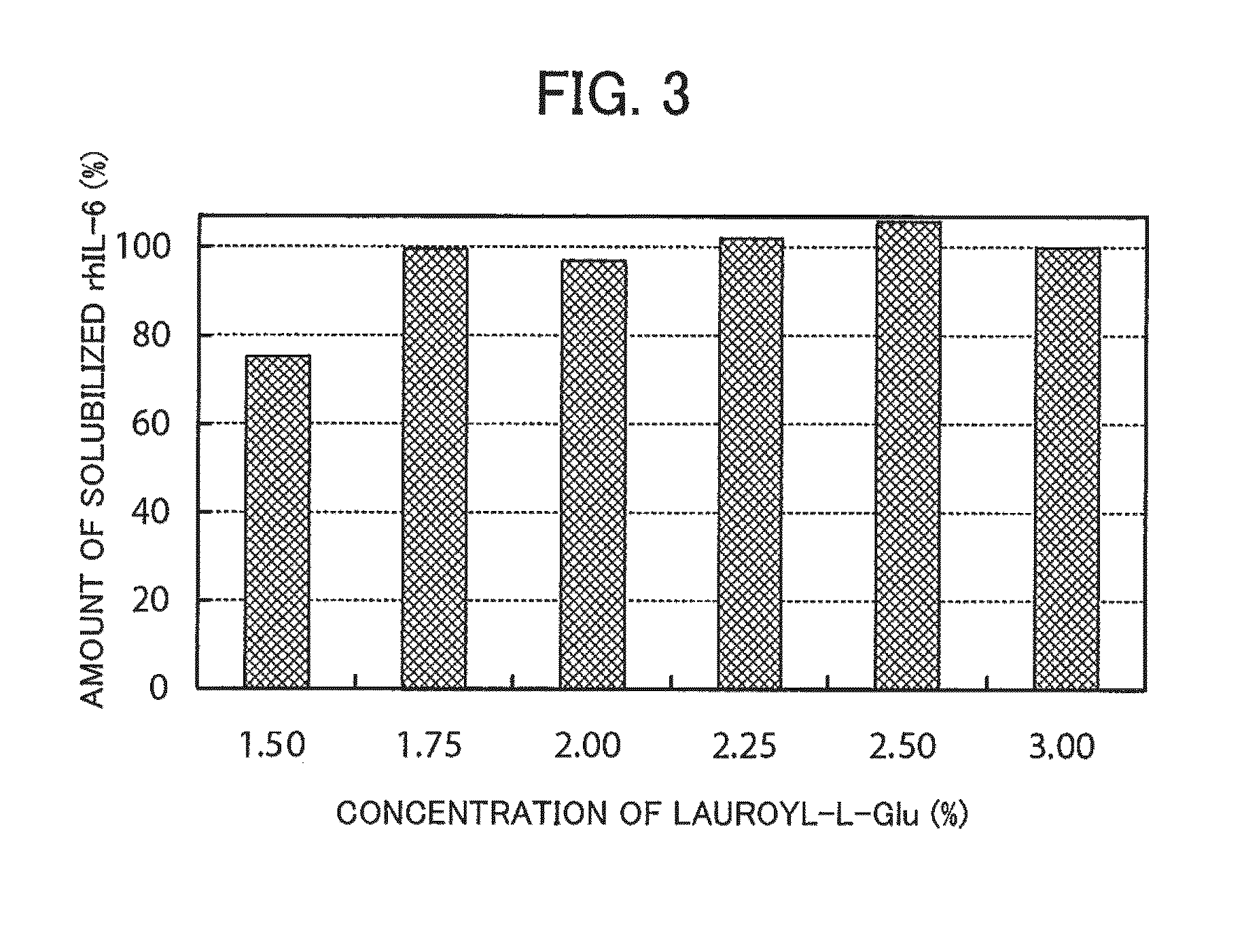
[0128]The concentration of surfactant for extracting rhIL-6 from the insoluble granules for solubilization was investigated.
[0129]Aliquots of 12.6 mg of the same water-insoluble granules of rhIL-6 as used in reference Example 1 were each put into an Eppendorf tube. A solution of lauroyl-L-Glu was added to the Eppendorf tubes to respectively achieve final concentrations of Lauroyl-L-Glu of 1.5%, 1.75%, 2.00%, 2.25%, and 3.00%, and thereby 3 ml of each solution of 10 mM sodium phosphate, pH 7.0 (25° C.) were added. The solutions were incubated at room temperature for 2 hours, thereby extracting rhIL-6 from the insoluble granules and solubilizing rhIL-6. If rhIL-6 was completely extracted, the concentration of the extraction of rhIL-6 would be adjusted to 4.3 mg / ml as a result.
[0130]After the extraction, 10 mM dithiothreitol (DTT) was added to a supernatant, and the supernatant containing DTT was adjusted to pH 8 and then heated at 37° C. for 30 minutes to reduce the disulfide bond. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| weight-average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com