Methods for Screening Inhibitors of Tau Phosphorylation By Casein Kinase I
a technology of casein kinase and inhibitors, which is applied in the field of screening inhibitors of tau phosphorylation by casein kinase i, can solve the problems of reducing the ability of tau to promote microtubule assembly and stabilise assembled microtubules, and achieves the effect of improving one or more of their properties and promoting dephosphorylation of tau proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
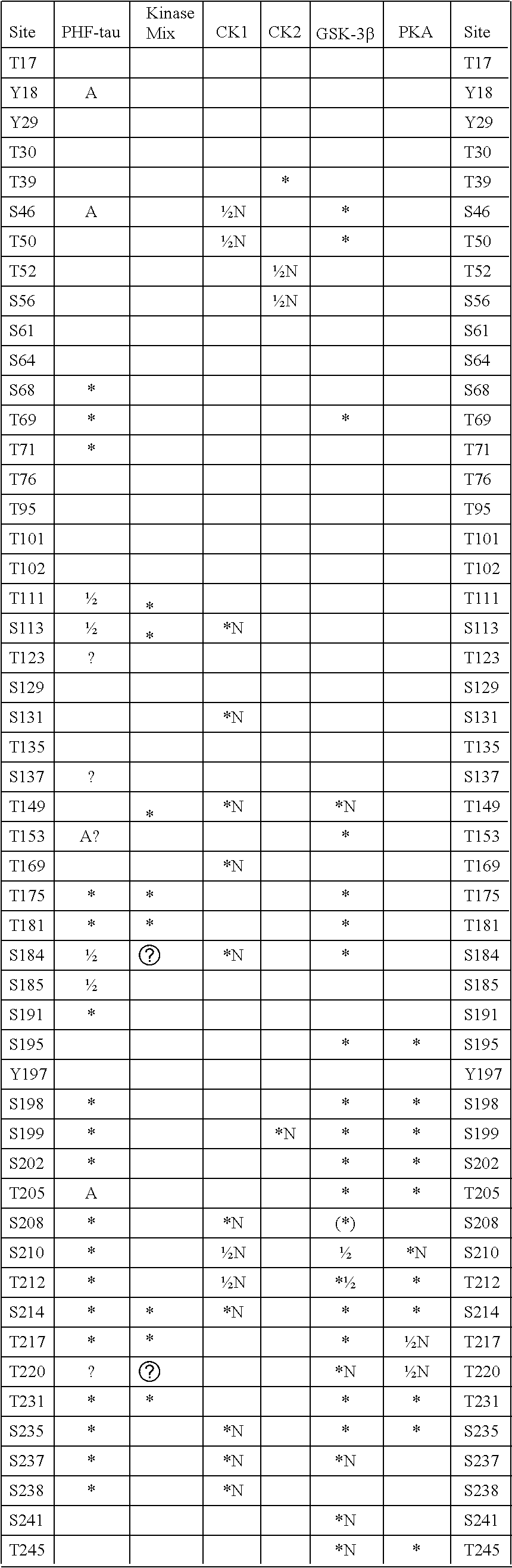
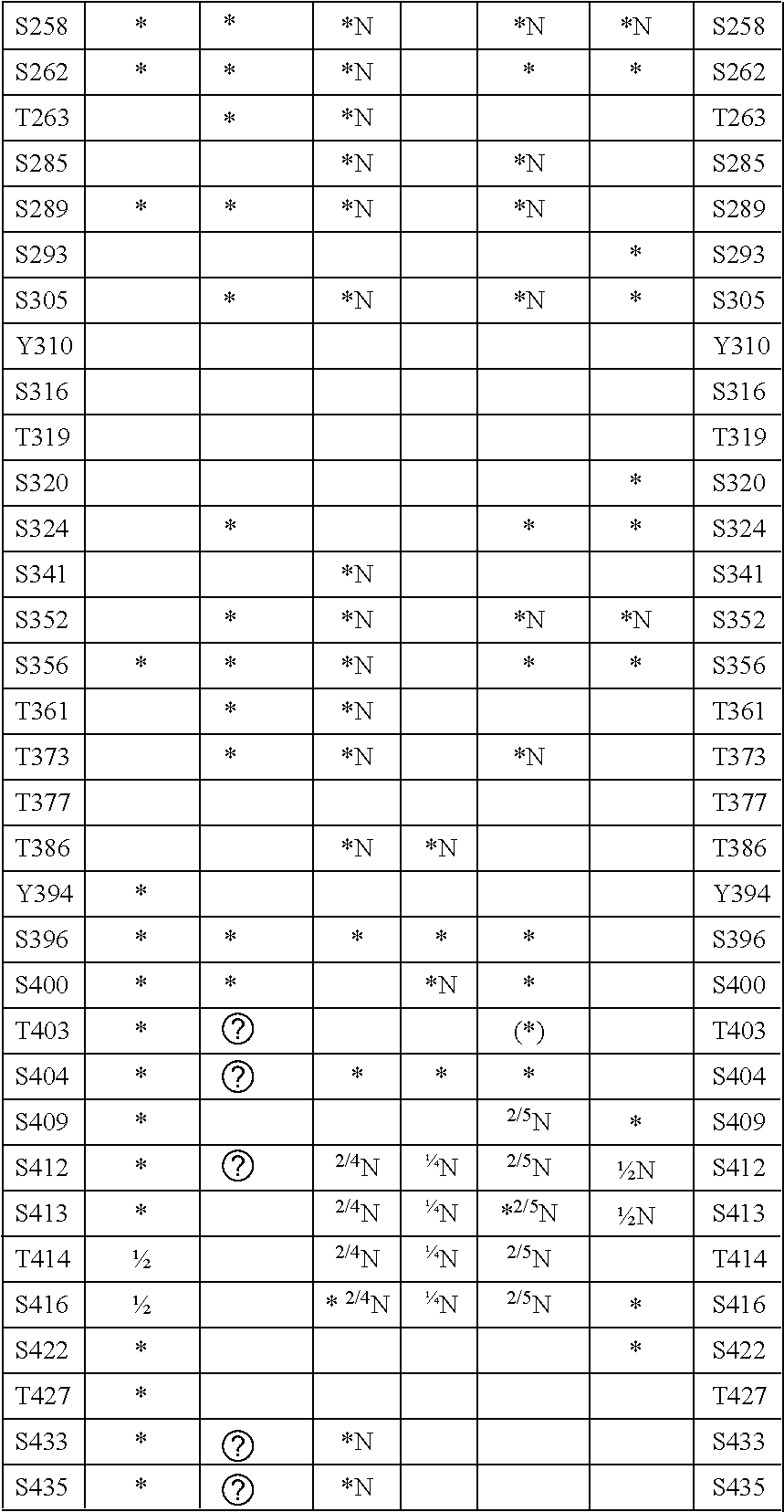
Tau Proteins
[0057]The assays and assay methods disclosed herein can employ wild-type or full length tau proteins, kinases or phosphatases or fragments, active portions or derivatives thereof. In the case of tau proteins, the materials used in the assays may be unphosphorylated or partially phosphorylated as discussed above.
[0058]In the present invention, derivatives of the tau proteins, kinases (especially CK1 kinase or fyn kinase) or phosphatases have an amino acid sequence which differs by one or more amino acid residues from the wild-type amino acid sequence, by one or more of addition, insertion, deletion and substitution of one or more amino acids. Thus, variants, derivatives, alleles, mutants and homologues, e.g. from other organisms, are included. Thus, a derivative of tau protein or CK1 kinase or fyn kinase may include 1, 2, 3, 4, 5, greater than 5, or greater than 10 amino acid alterations such as substitutions with respect to the wild-type sequence.
[0059]Preferably, a frag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| PKA | aaaaa | aaaaa |
| PKA | aaaaa | aaaaa |
| PKA | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com