Application of targeting STAT1 inhibitor in treatment of immune thrombocytopenia and method
A technology of thrombocytopenia and application method, applied in the field of biomedicine, can solve the problem of increased risk of bacterial infection, and achieve the effect of treating immune thrombocytopenia, high bioavailability and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
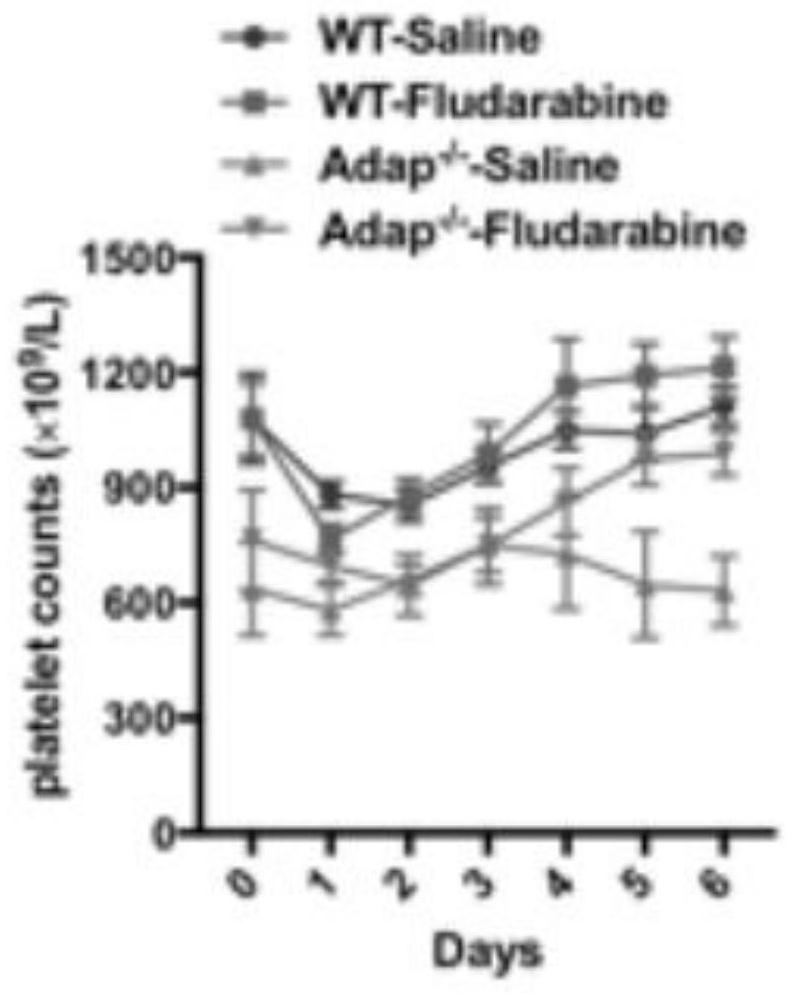
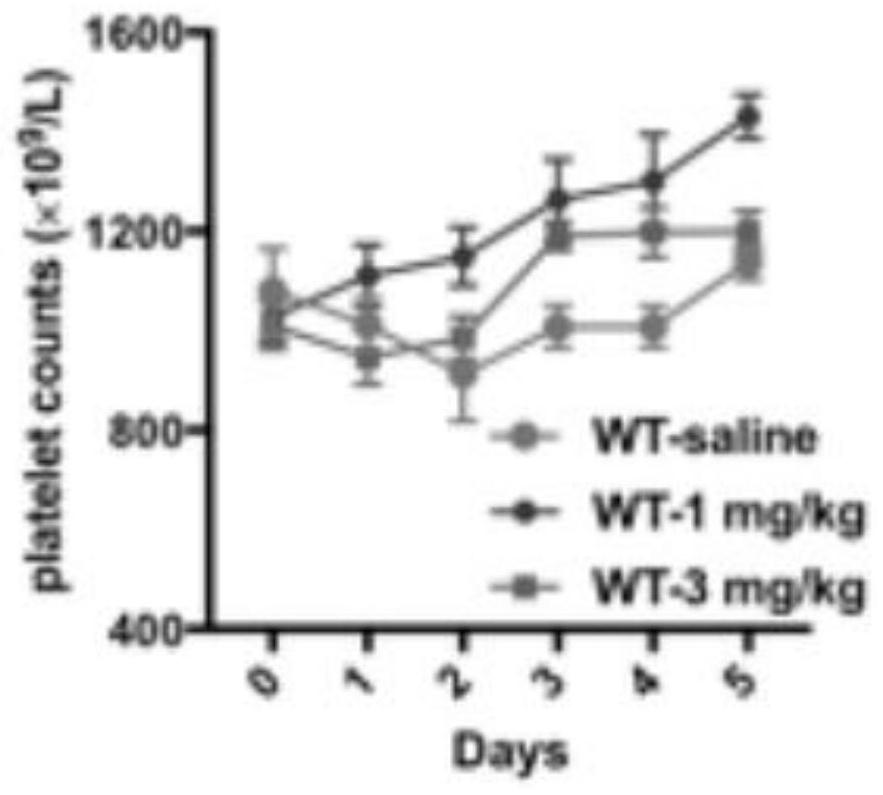
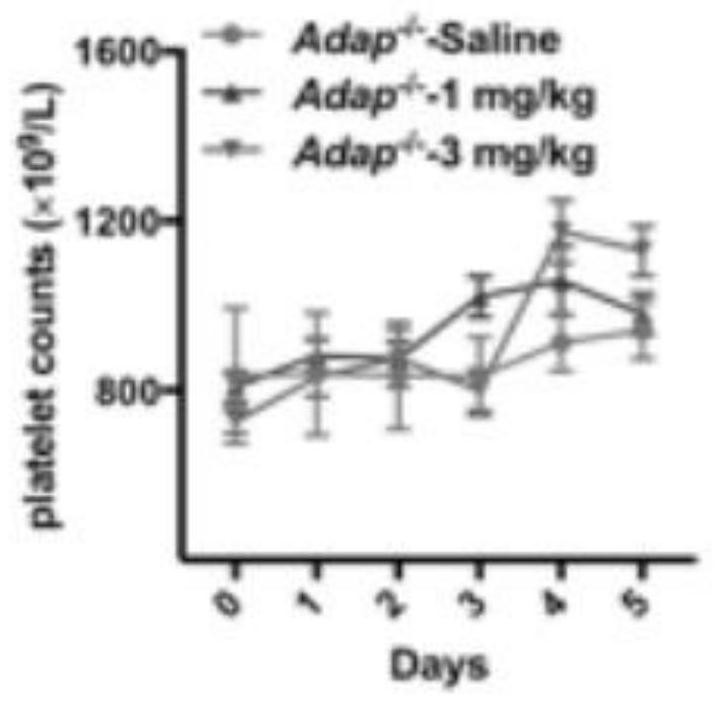
[0040] An application of a STAT1 nuclear import / activation inhibitor in the treatment of immune thrombocytopenia, the application is that the STAT1 activation inhibitor increases the number of platelets, and the inhibitor is fludarabine.
Embodiment 2
[0042] An application of a STAT1 nuclear import / activation inhibitor in the treatment of immune thrombocytopenia, the application is that the STAT1 nuclear import inhibitor increases the number of platelets, and the inhibitor is ivermectin.
Embodiment 3
[0044] A method for preparing a fludarabine solution for treating immune thrombocytopenia by using a STAT1 activation inhibitor, comprising the steps of:
[0045] S1: Weigh the STAT1 activation inhibitor fludarabine;
[0046] S2: dissolving the STAT1 activation inhibitor fludarabine weighed in step S1 in DMSO;
[0047] S3: use physiological saline to dilute the DMSO in step S2,
[0048] Wherein, the final volume content of DMSO in the diluted solution is 0-5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com