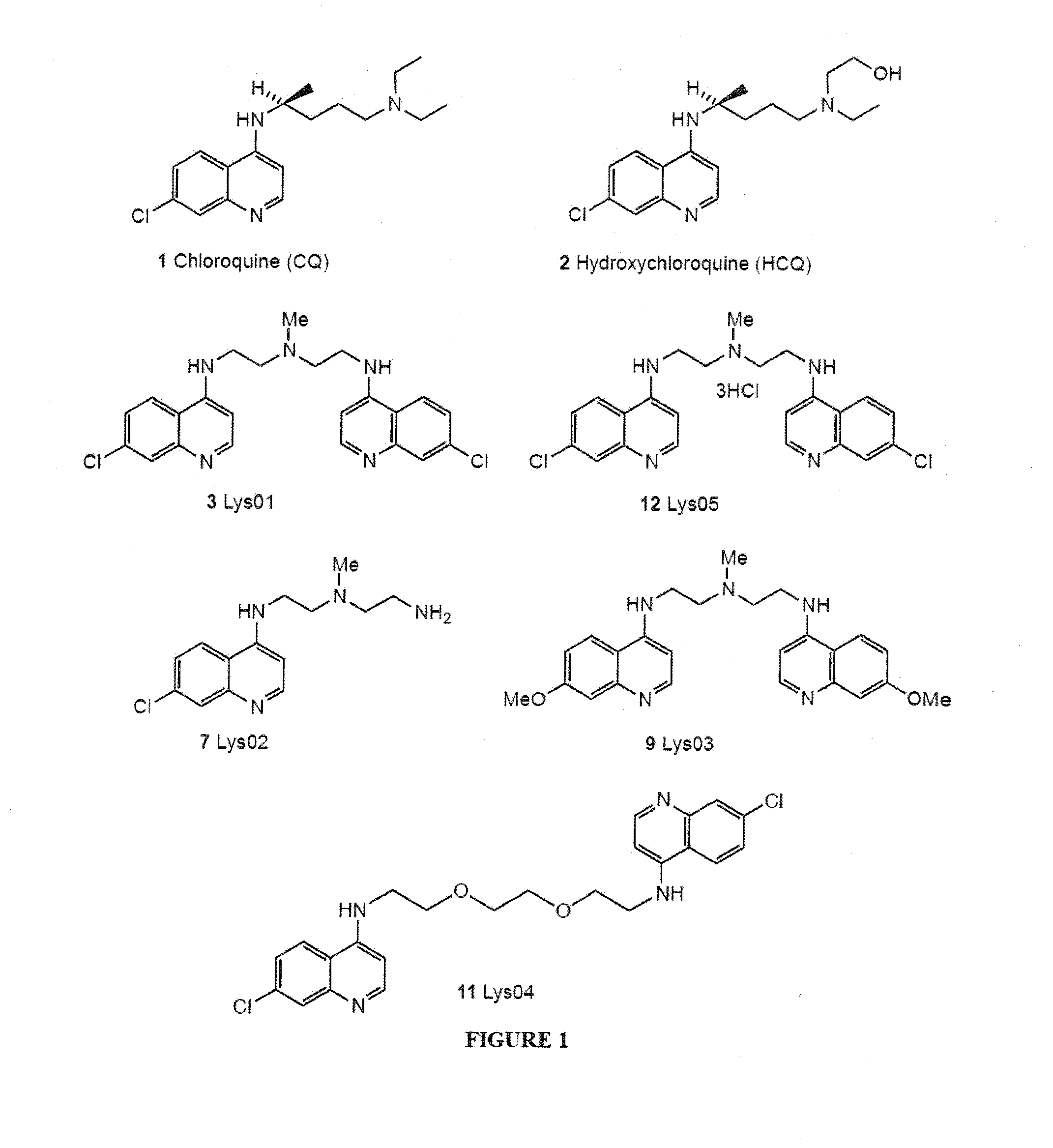
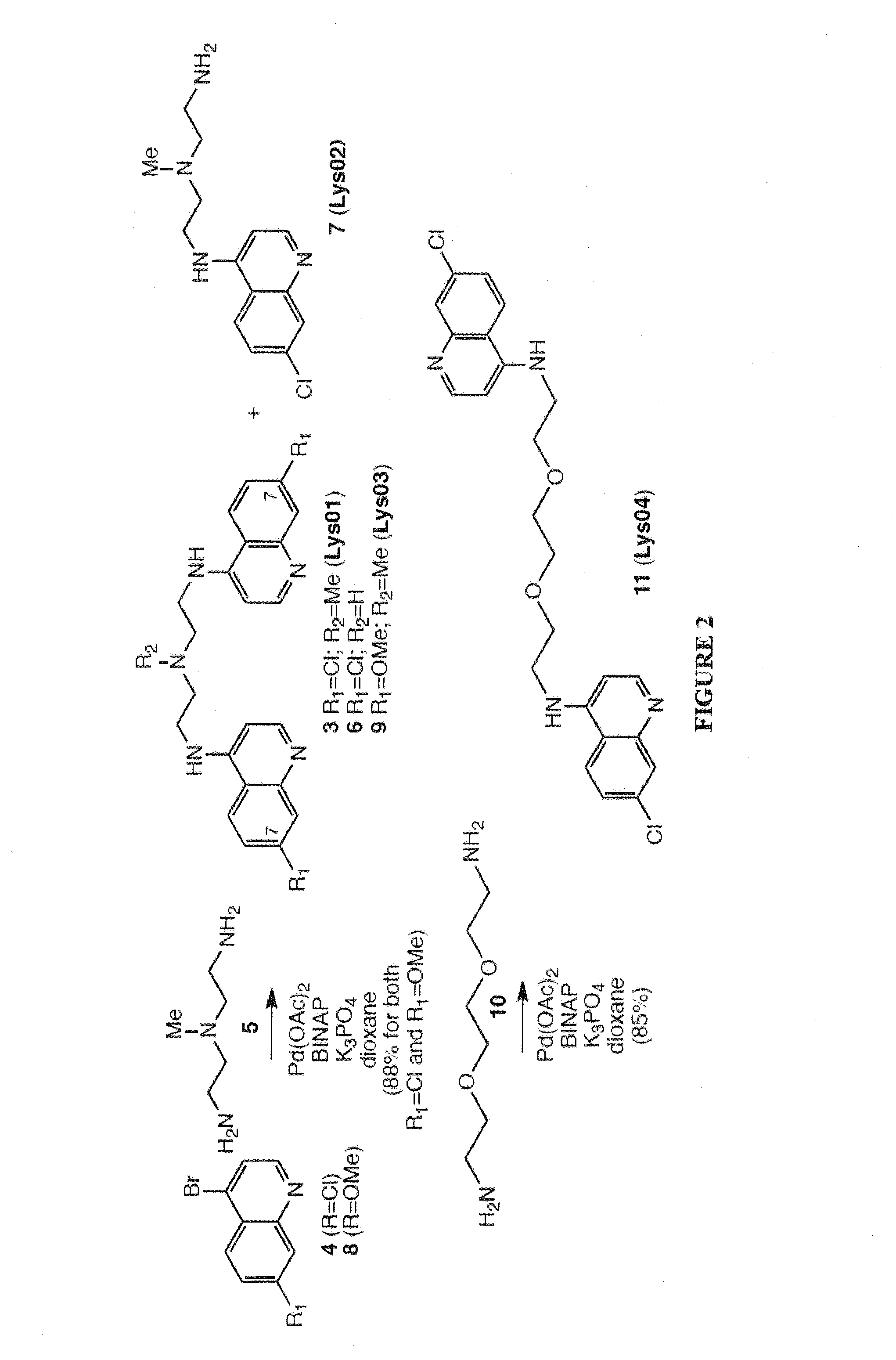
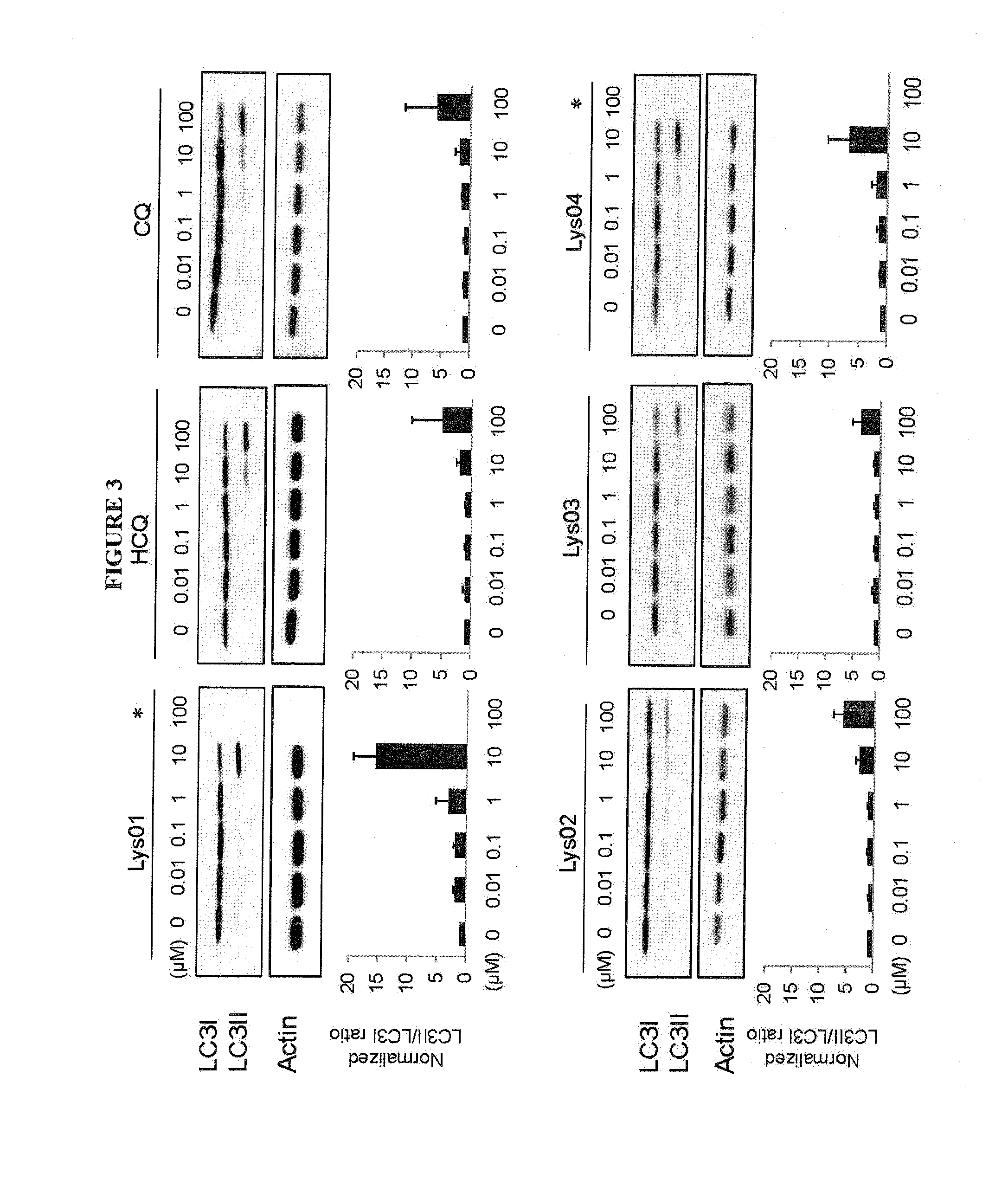
Novel bisaminoquinoline compounds, pharmaceutical compositions prepared therefrom and their use
a technology of bisaminoquinoline and compound, which is applied in the field of new bisaminoquinoline compounds, can solve the problems that none of the compounds had sufficient antimalarial activity to warrant further investigation, and achieve the effect of inhibiting autophagy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0104]The following examples illustrate and describe the present invention but are not intended to limit the invention in any way.
[0105]Synthesis of Compound 3 (Lys01).
[0106]A round-bottom flask was charged with the 4-bromo-7-chloroquinoline (compound 5) (734 mg, 3.0 mmol), Pd(OAc)2 (23 mg, 0.1 mmol), BINAP (125 mg, 0.2 mmol), K3PO4 (1.06 g, 5.0 mmol), and triamine (compound 6) (117 mg, 1.0 mmol). Dioxane (10 mL) was introduced through the septum. The resulting suspension was stirred under argon at 90° C. for 18 h and cooled. The mixture was adsorbed onto silica gel and purified by flash chromatography (CH2Cl2 / MeOH: 90 / 9 / 1) to afford compound 3 (387 mg, 88%) as a yellow solid. mp 199-200° C.; Rf=0.28 (silica gel, CH2Cl2 / MeOH / NH4OH: 90 / 9 / 1); 1H NMR (500 MHz, CDCl3:δ 8.53 (d, J=5.5 Hz, 2H), 7.94 (d, J=2.0 Hz, 2H), 7.41 (d, J=9.0 Hz, 2H), 6.98 (dd, J=9.0, 2.0 Hz, 2H), 6.39 (d, J=5.0 Hz, 2H), 5.44 (s, 2H). 3.42 (q, J=5.0 Hz, 4H), 2.90 (t, J=6.0 Hz, 2H), 2.46 (s, 9H). 13C NMR (125 MHz, C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| chemical structure | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com