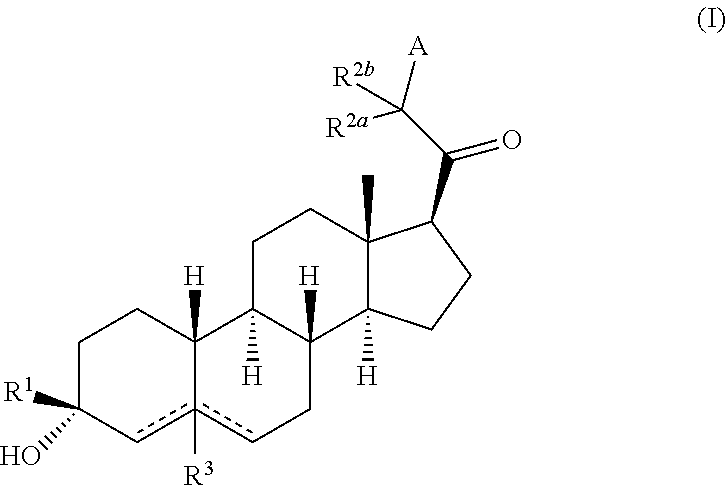
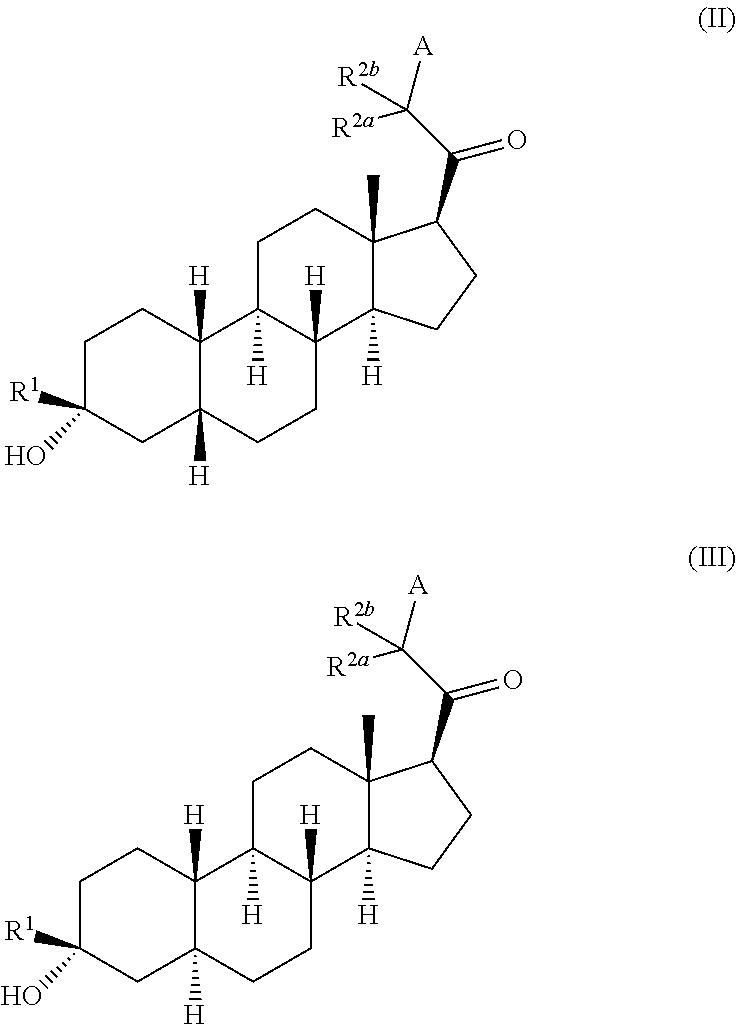
Compositions and methods for treating CNS disorders
a technology for cns disorders and compositions, applied in the field of compositions and methods for treating cns disorders, can solve the problems that progesterone is not consistently effective in the treatment of the aforementioned syndromes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of 1 and 2
[0258]
[0259]To a solution of A1 (500 mg, 1.29 mmol) in THF (4 mL was added KOH (144 mg, 2.58 mmol) and MeI (200 mg, 1.41 mmol) at 25° C. The mixture was stirred at 25° C. for 2 h. After TLC showed the starting material was consumed, the reaction mixture was treated with water (20 mL) and extracted with EtOAc (30 mL×2). The organic phase was washed with brine (30 mL), dried over anhydrous Na2SO4, concentrated in vacuum. The residue was purified by prep-HPLC to afford (R)-1-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxy-3,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)-2-(2H-1,2,3-triazol-2-yl)propan-1-one (79.5 mg, 15.4%) and (S)-1-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxy-3,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)-2-(2H-1,2,3-triazol-2-yl)propan-1-one (93.1 mg, 18%) as white solid.
[0260]1H NMR (1) (400 MHz, CDCl3) δ 7.67 (s, 2H), 5.27-5.22 (m, 1H), 2.25-2.23 (m, 1H), 2.11-2.05 (m, 1H), 1.87-1.66 (m, 9H), 1.41-1.02 (m, 19H), 0.65 (s, 3H). LCMS Rt=0.9...
example 2
of 3 and 4
[0262]
To a solution of A2 (400 mg, 0.976 mmol) in THF (3 mL) was added KOH (109 mg, 1.95 mmol) and MeI (1.58 g, 11.1 mmol) at 25° C. The mixture was stirred at 25° C. for 2 h. After TLC showed the starting material was consumed, the reaction mixture was treated with water (20 mL) and extracted with ELOAc (30 mL×2), The organic phase was washed with brine (30 mL), dried over anhydrous Na2SO4, concentrated in vacuum. The residue was purified by column chromatography on silica gel (PE / EtOAc=5 / 1 to EtOAc) to afford 1-((S)-1-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxy-3,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)-1-oxopropan-2-yl)-1H-pyrazole-4-carbonitrile (100 mg, 24.2%) and 1-((R)-1-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxy-3,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)-1-oxopropan-2-yl)-1H-pyrazole-4-carbonitrile (139 mg, 33.6%) as a white solid.
[0263]1H NMR (3) (400 MHz, CDCl3) δ 7.99 (s, 1H), 7.77 (s, 1H), 5.27-5.25 (m, 1H), 2.73-2.71 (m, 1H), 2...
example 3
of 5
[0265]
[0266]Step 1. Synthesis of A4.
[0267]To a solution of 2,6-di-tert-butyl-4-methylphenol (A3, 24 g, 109 mmol) in toluene (100 mL) was added AlMe3 (2 M in toluene, 27.3 mL, 54.6 mmol) dropwise at 10° C. The mixture was then stirred at 25° C. for 1 hour. To the mixture was added a solution of compound (5R,8R,9R,10S,13S,14S)-13-methyldodecahydro-1H-cyclopenta[a]phenanthrene-3,17(2H,4H)-dione (5 g, 18.2 mmol) in toluene (50 mL) dropwise at −70° C. dropwise under N2. The mixture was stirred at −70° C. for 1 hour. MeMgBr (3 M in ether, 18.2 mL, 54.6 mmol) was added dropwise at −70° C. The mixture was stirred at −70° C. for another 3 hours. TLC showed the reaction was completed. The mixture was poured into citric acid (150 mL, 20% aq.). The mixture was extracted with EtOAc (100 mL*2). The combined organic layer was concentrated under vacuum, purified by column chromatography on silica gel (petroleum ether:EtOAc=50:1 to 1:1) to give (3R,5R,8R,9R,10S,13S,14S)-3-hydroxy-3,13-dimethylte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| membrane voltage | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com