Neuroactive steroids, compositions, and uses thereof
a technology of compositions and neuroactive steroids, applied in the field of neuroactive steroids, compositions, can solve the problems that progesterone is not consistently effective in the treatment of the aforementioned syndromes, and achieve the effects of reducing or avoiding symptoms or causes of disease, and reducing or minimizing one or more symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compounds 1 and 2
[0248]Synthesis of A1 and A2 Intermediates
[0249]Synthesis of Compounds 1 and 2
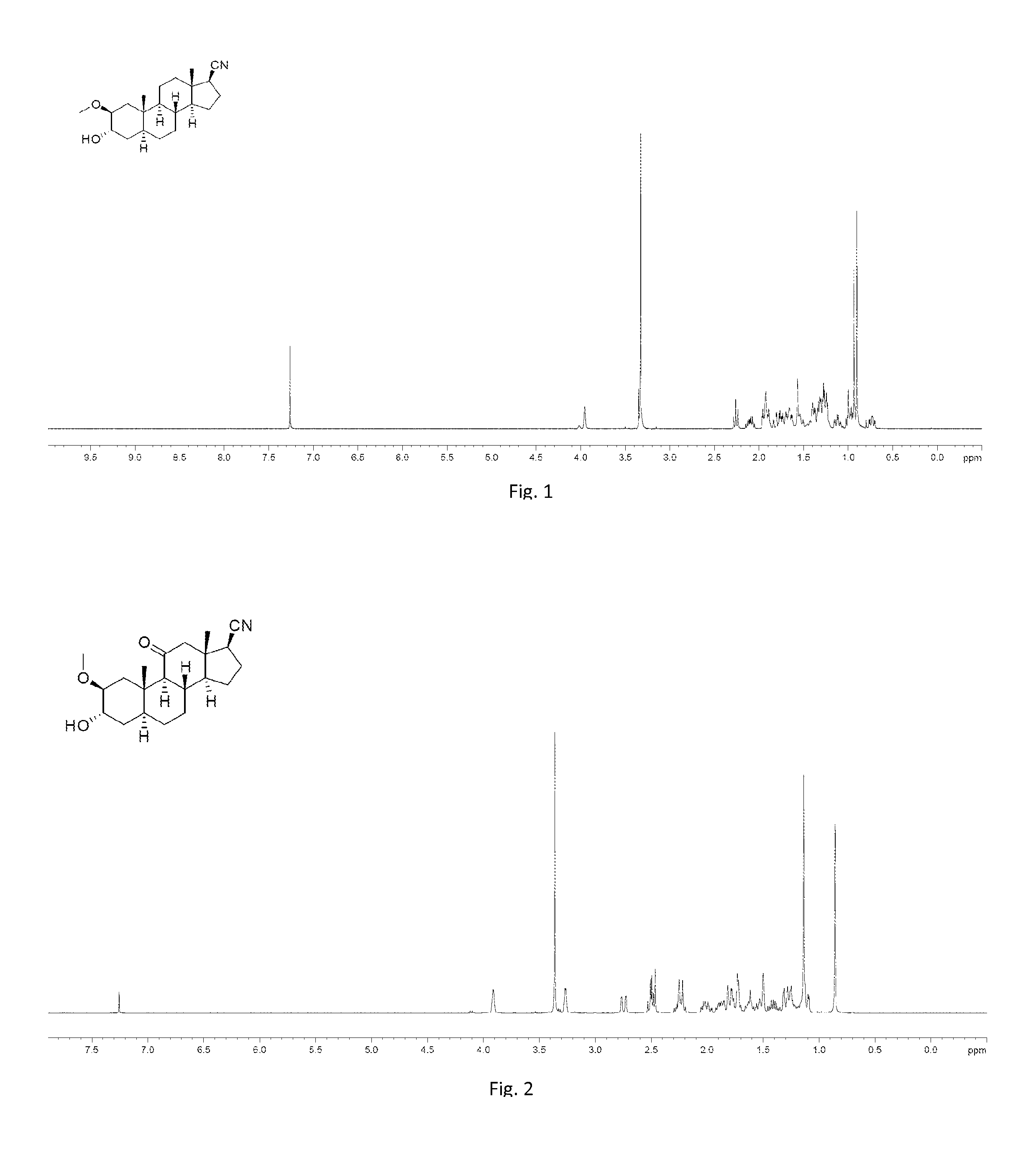
[0250]To a solution of 006-1, dehydroepiandrosterone (11 g, 38.17 mmol) in EtOH (150 mL) was added 10% Pd / C (1.1 g). Then the mixture was stirred under H2 (40 psi) at 40° C. for 12 hours. TLC showed the starting material was consumed completely. The mixture was filtered and the filtrate was concentrated under vacuum to give 008-1 (11 g, 99%) as a white solid. 1H NMR (008-1): (400 MHz, CDCl3) δ 3.59-3.51 (m, 1H), 2.46-2.39 (m, 1H), 2.10-2.01 (m, 1H), 1.92-1.81 (m, 1H), 1.80-0.94 (m, 19H), 0.85 (s, 3H), 0.82 (s, 3H), 0.69-0.62 (m, 1H).
[0251]To a solution of 008-1 (11 g, 37.8 mmol) in dry pyridine (150 mL) was added p-TsCl (11.4 g, 68.2 mmol) in portions. The mixture was stirred at 40° C. for 6 hours. Water was added slowly, then a white solid precipitated. The white solid was filtered, and washed with aqueous HCl (200 mL*3, 1M), followed by water (200 mL*3). The solid was dried ...
example 2
Synthesis of Compounds 3 and 38
[0256]
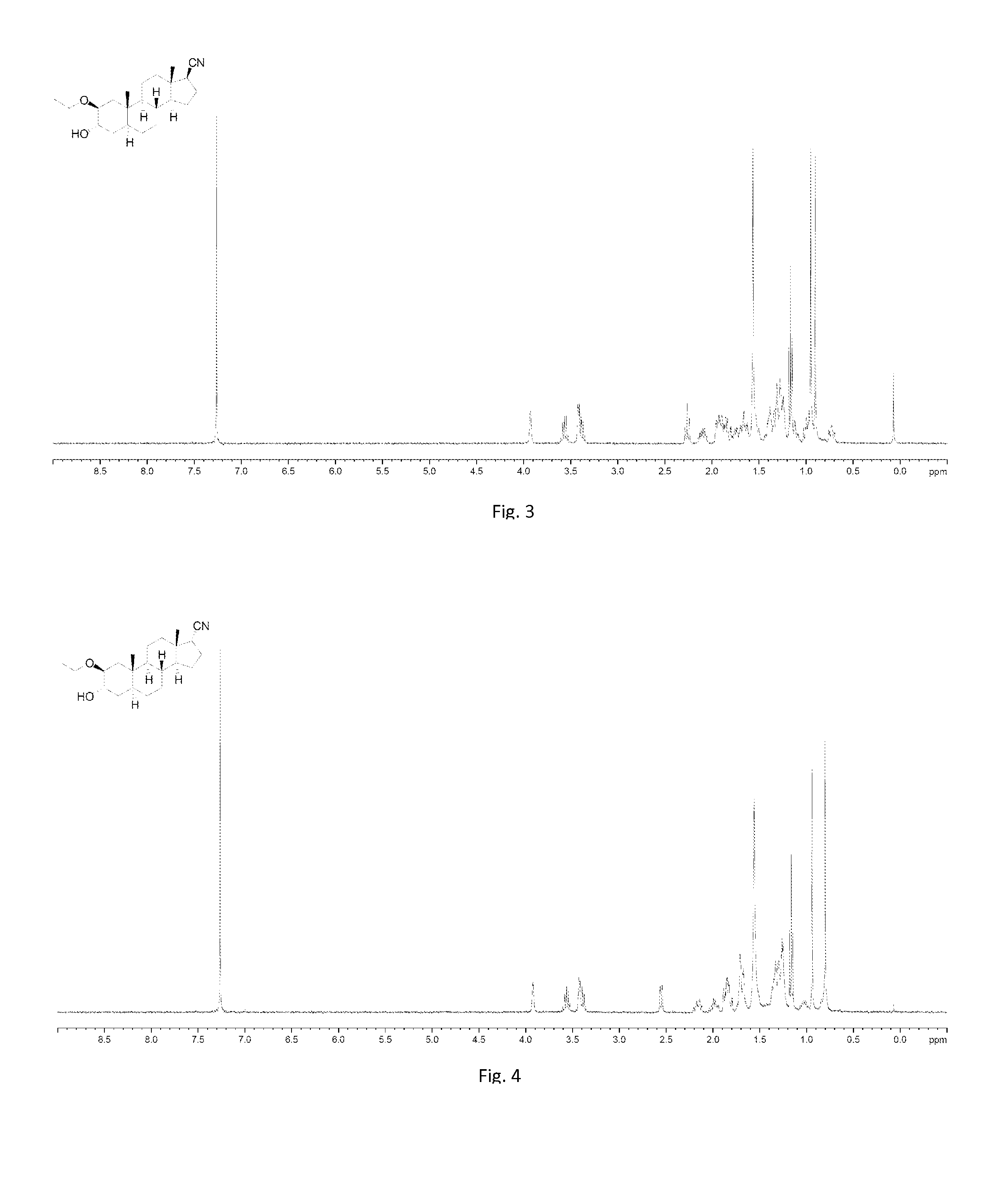
[0257]To a solution of 001-1 (20 g, 55 mmol) in pyridine (200 mL) was added dropwise Ac2O (8.45 g, 82.8 mmol), then the mixture was stirred at room temperature over night. TLC (petroleum ether:ethyl acetate=1:1) showed that the starting material was consumed completely. The mixture was poured into water (1.5 L) with stirring. The resulting solid was collected by filtration and washed with 500 mL of HCl (1 M), followed by water (500 mL×3). The solid was dried by lyophilization, and 001-2 (19.1 g, 85.9%) was obtained as a white solid. 1H NMR (001-2): (400 MHz, CDCl3) δ 5.68 (s, 1H), 5.03 (d, J=17.6 Hz, 1H), 4.84 (d, J=17.6 Hz, 1H), 4.48-4.47 (m, 1H), 2.81-2.77 (m, 1H), 2.57-2.20 (m, 5H), 2.17 (s, 3H), 2.17-1.97 (m, 3H), 1.91-1.79 (m, 2H), 1.76-1.64 (m, 2H), 1.54-1.46 (m, 2H), 1.42 (s, 3H), 1.30-1.07 (m, 3H), 0.98 (s, 3H), 0.90-0.89 (m, 1H).
[0258]To a solution of 001-2 (17 g, 42 mmol) in EtOH (19 mL) and THF (190 mL) was added CH(OEt)3 (38.6 mL, 231...
example 3
Synthesis of Compounds 47 and 48
[0268]Synthesis of Intermediates 039-1 and 051-1
[0269]Synthesis of Compounds 47 and 48
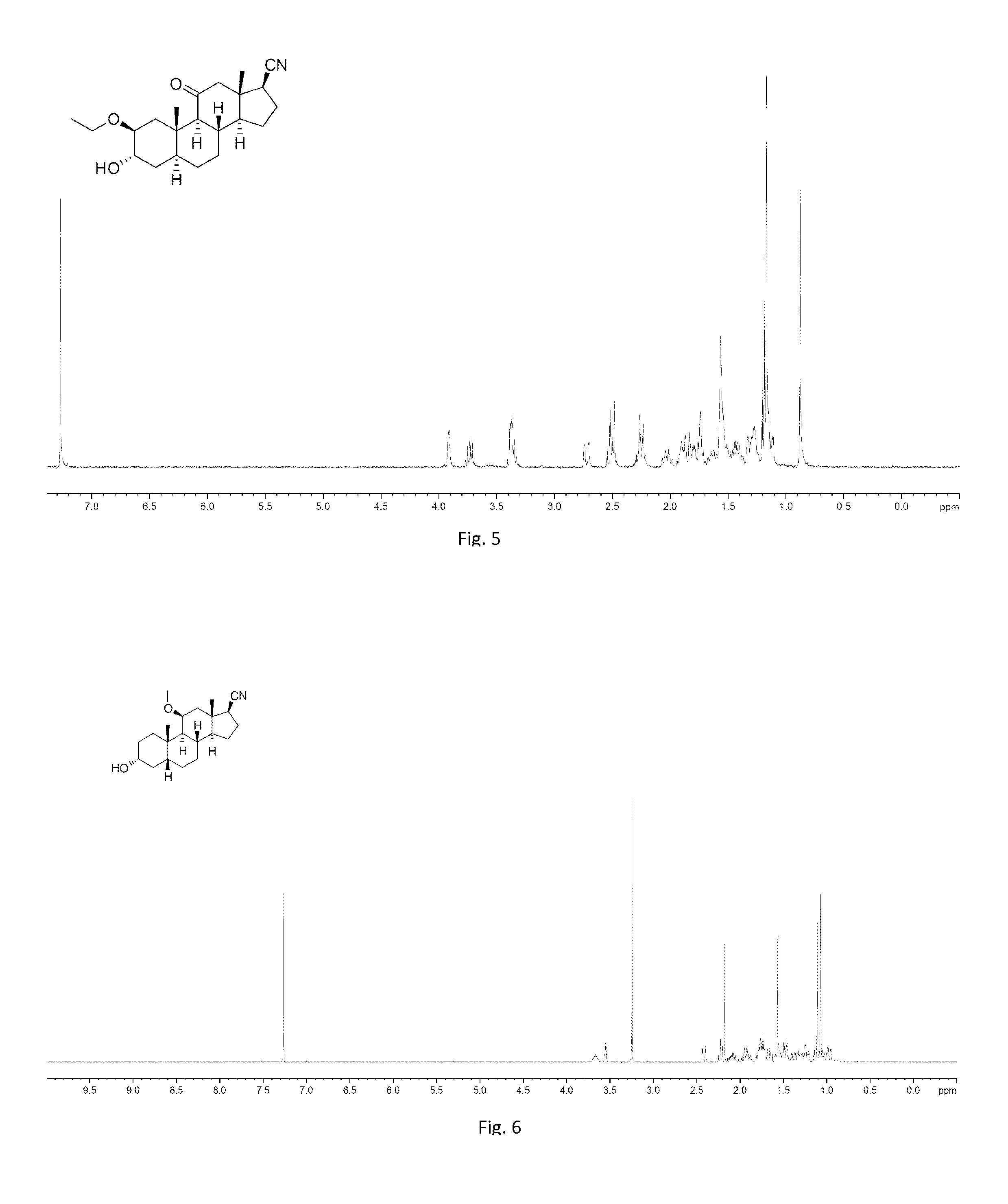
[0270]To a solution of the mixture of 008-4 and 008-4A (2.5 g, 8.68 mmol) in EtOH (75 mL) was added H2SO4 (10 drops, 98%). The mixture was stirred at 20° C. for 3 h. TLC showed the starting material was consumed completely. The mixture was quenched with aqueous NaHCO3 (40 mL). The mixture was extracted with EtOAc (100 mL×2) and washed with aqueous NaCl (50 mL). The organic phase was dried over Na2SO4 and evaporated to give the crude product, which was purified by column chromatography on silica gel (ethyl acetate: petroleum ether=1:2) to afford the mixture of 039-1 and 051-1 (1.8 g, 60%) as a white solid.
[0271]To a solution of t-BuOK (1.47 g, 13.16 mmol) in t-BuOH (10 mL) was added a solution of 039-1 (440 mg, 1.32 mmol) in 1,2-dimethoxyethane (4 mL) dropwise at room temperature. Then a solution of TosMic (1.0 g, 5.1 mmol) in 1,2-dimethoxyethane (6 mL) was added drop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| membrane voltage | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| δ (ppm) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com