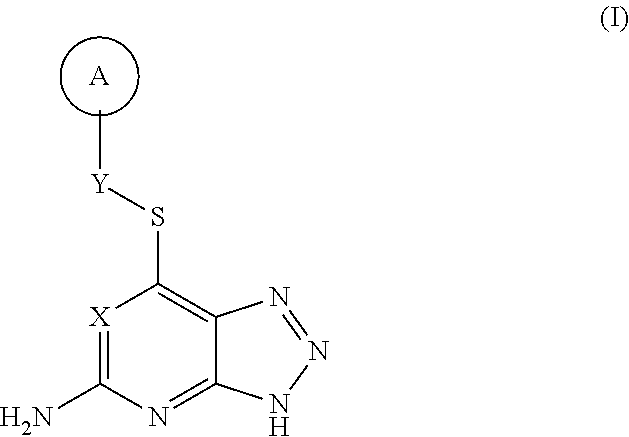
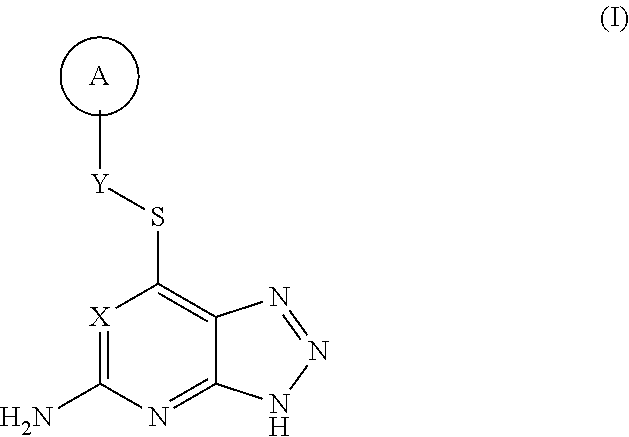
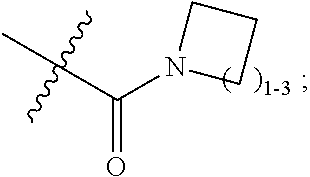
Thioether triazolopyridine and triazolopyrimidine inhibitors of myeloperoxidase
a technology of triazolopyrimidine and thioether triazolopyridine, which is applied in the direction of drug compositions, organic chemistry, cardiovascular disorders, etc., can solve the problem that cardiovascular disease is a major health risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
hio)-3H-[1,2,3]triazolo[4,5-b]pyridin-5-amine
[0315]
[0316]To a mixture of Intermediate 2 (15 mg, 0.088 mmol), and phenylmethanethiol (22 mg, 0.18 mmol) in DMSO (1 mL) was added cesium carbonate (58 mg, 0.18 mmol), and the reaction mixture was stirred at 85° C. for 10 h. The crude mixture was filtered, and the filtrate was diluted with MeOH and purified by preparatory HPLC to obtain the title compound (19 mg, 0.069 mmol, 78% yield) as a yellow solid. MS (ESI) m / z 258.2 (M+H). 1H NMR (500 MHz, CD3OD) δ 8.25 (d, J=7.2 Hz, 2H), 8.21-8.00 (m, 3H), 7.45 (br. s., 1H), 5.34-5.22 (m, 2H).
[0317]The following compounds were prepared in a manner similar to experimental procedures that are described in Example 1 using Intermediate 2 and the appropriate thiol as starting materials:
Ex.No.StructureNameAnalytical Data27-((1-phenylethyl)thio)- 3H-[1,2,3]triazolo[4,5- b]pyridin-5-amineMS (ESI) 272.1 (M + H)+. 1H NMR (500 MHz, CD3OD)) δ 7.55-7.49 (m, 2H), 7.39- 7.33 (m, 2H), 7.31-7.24 (m, 1H), 6.63 (s, ...
example 10a
hlorophenyl)diazenyl)-4-((2,6-difluorobenzyl)thio)pyridine-2,6-diamine
[0319]
[0320]A mixture of Intermediate 2A, (2,6-difluorophenyl)methanethiol (230 mg, 1.4 mmol) and Cs2CO3 (1800 mg, 5.7 mmol) in DMSO (2 mL) was heated for 1 h at 90° C. The mixture was diluted with water, and the mixture was extracted with EtOAc. The combined organics were washed with 1N HCl and brine, dried over MgSO4 and concentrated. The crude was purified by column chromatography to yield Example 10A as an impure brown oil. MS (ESI) m / z 406.2 / 408.2.
example 10b
luorobenzyl)thio)pyridine-2,3,6-triamine
[0321]
[0322]To a solution of 10A (700 mg, 1.7 mmol) in MeOH (50 mL) was added zinc powder (340 mg, 5.2 mmol) followed by glacial acetic acid (0.296 mL, 5.17 mmol). The resulting reaction mixture was heated at 60° C. for 2 h. The reaction mixture was cooled to rt and filtered through a pad of celite. The filtrates were combined and concentrated. The crude product was dissolved in 5.0 mL of 7.0 M NH3 in MeOH and concentrated. The crude product was purified by column chromatography (2.0 M NH3 in MeOH / CH2Cl2, 0-20% gradient) to yield Example 10B (300 mg, 1.1 mmol, 62% yield). MS (ESI) m / z 283.3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com