Treatment of cancer by infusion of oncolytic herpes simplex virus to the blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
—Cytokine Responses Following Intrapleural Administration of Oncolytic HSV SEPREHVIR® in Patients with Malignant Pleural Mesothelioma
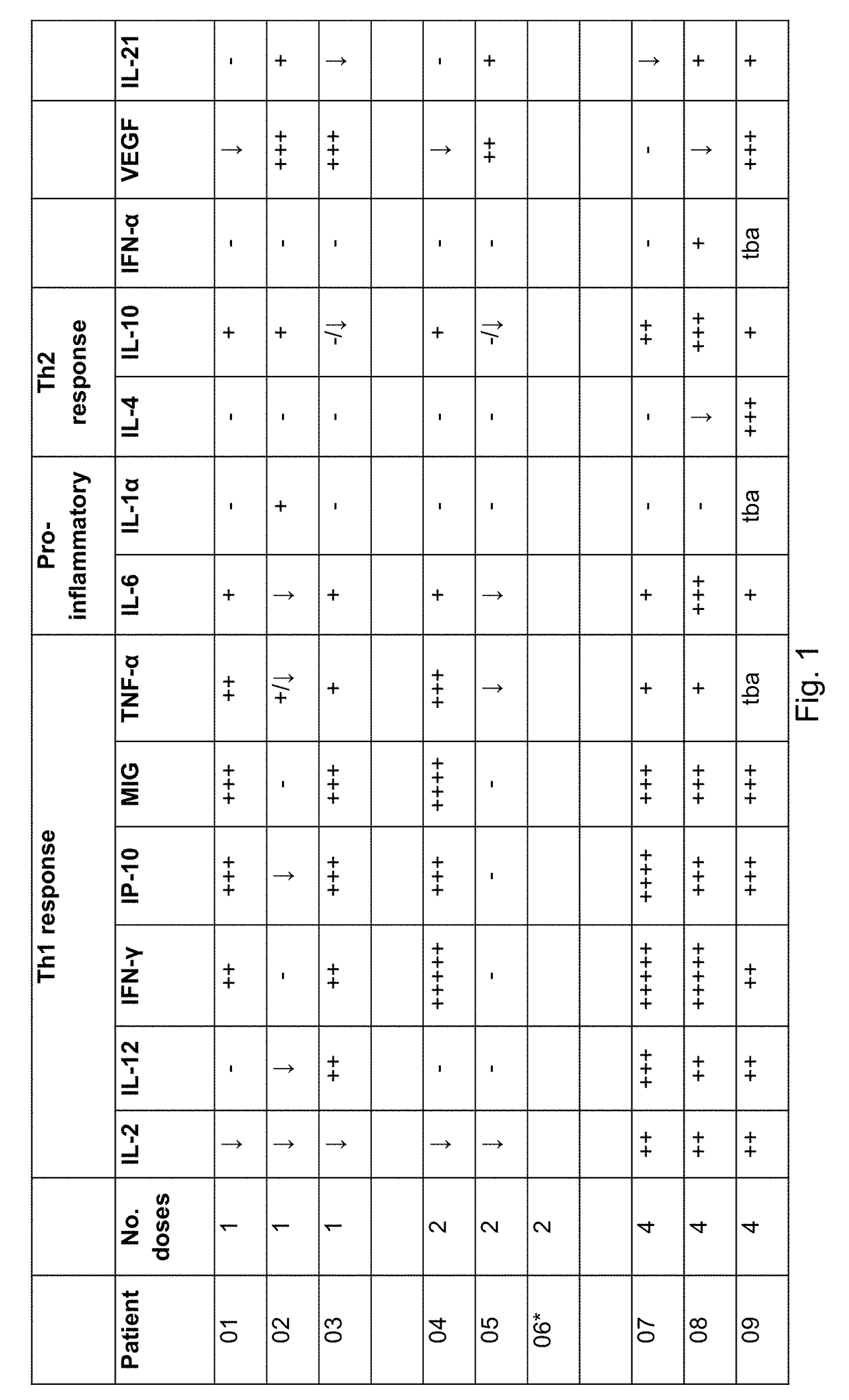
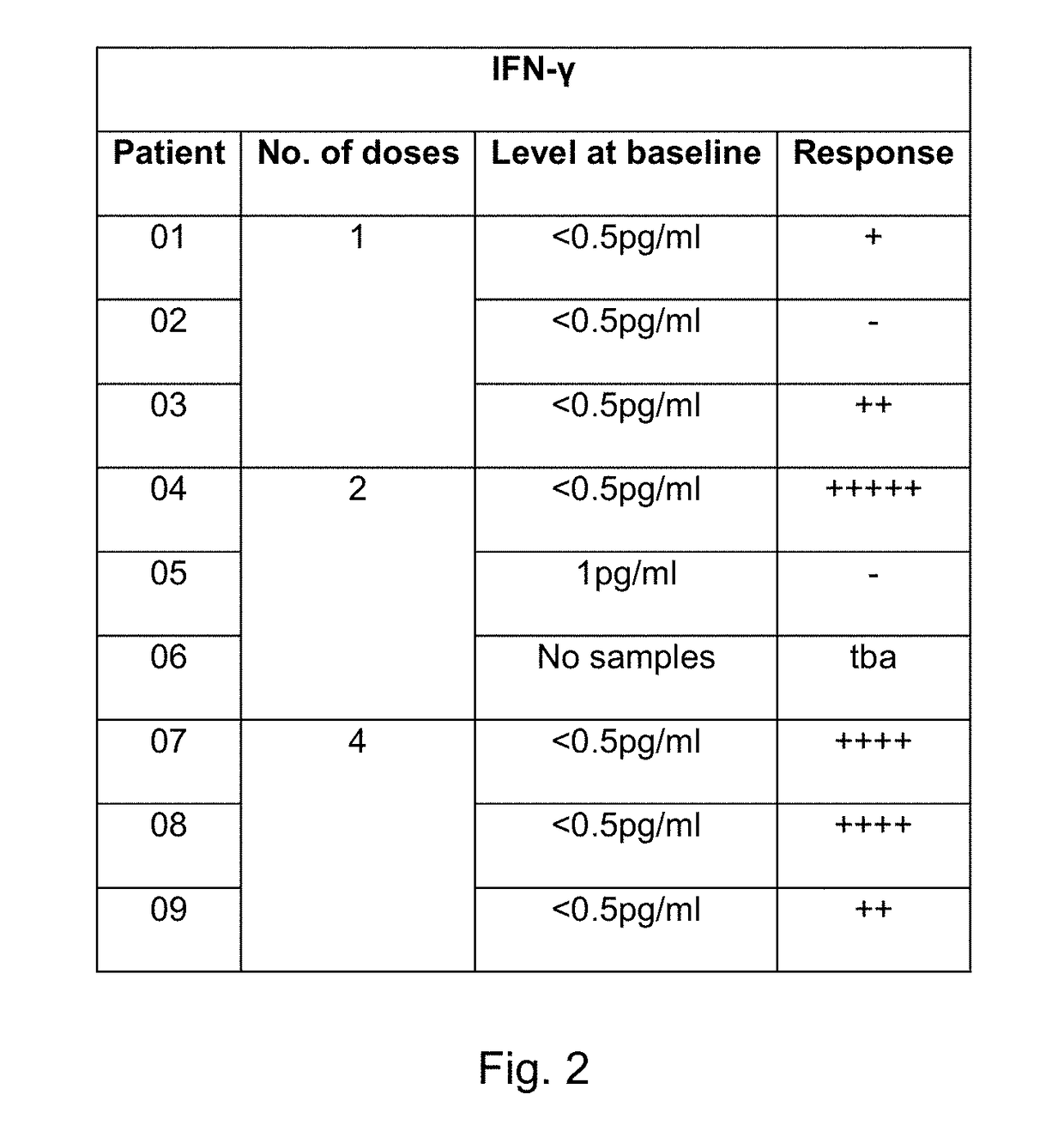
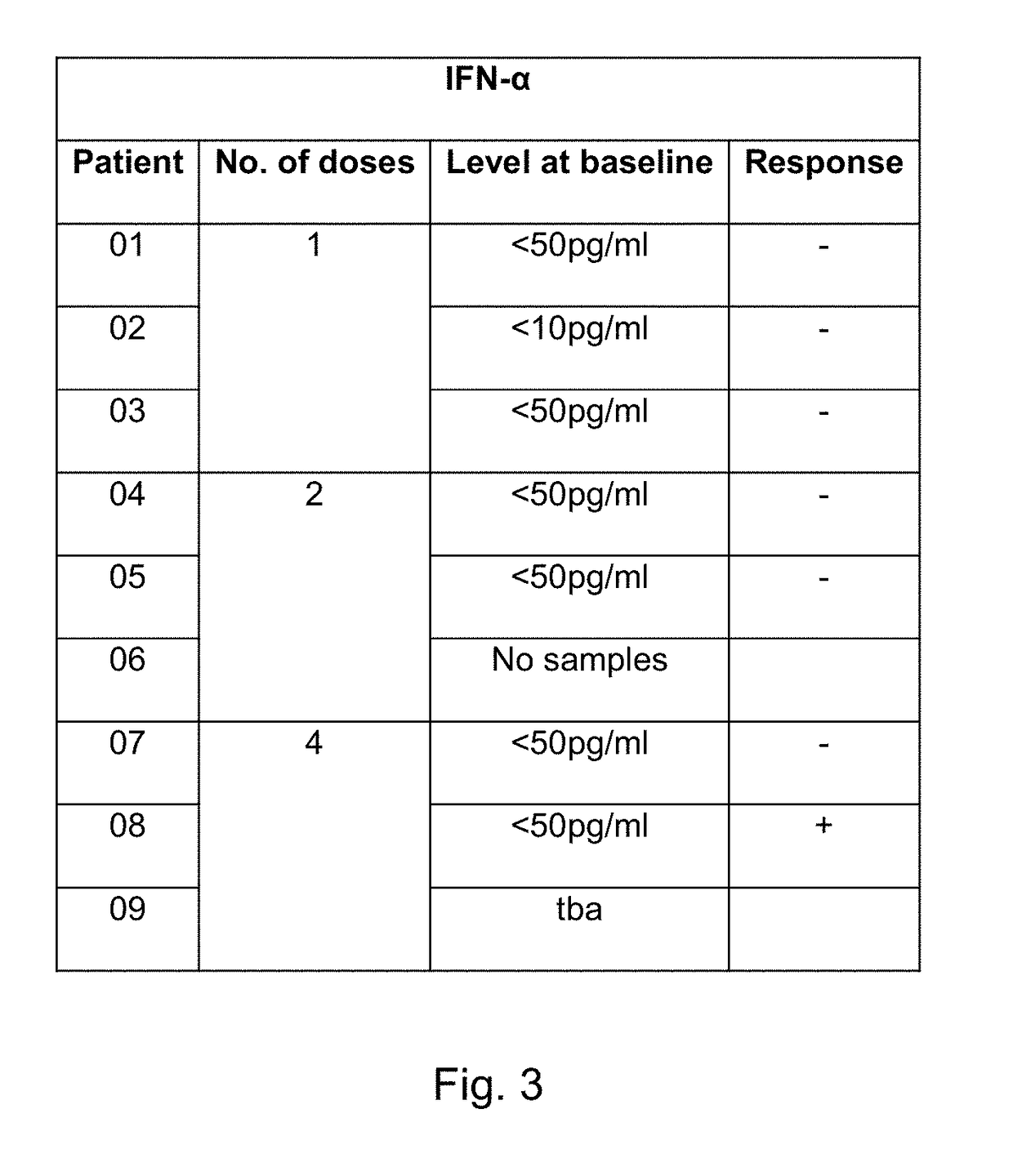
[0584]We are currently conducting a phase I / 11a trial at Cancer Clinical Trials Centre, Weston Park Hospital, Sheffield and Queen Elizabeth University Hospital, Glasgow, United Kingdom to determine the safety and potential for efficacy of SEPREHVIR® given intrapleurally to patients with malignant pleural mesothelioma (MPM). Patients receive 1×107 iu SEPREHVIR® through their pleural catheter on one, two or four occasions each dose given one week apart, in three separate patient cohorts. To date 11 patients have been treated, 3 in each 1 and 2 dose cohorts and 5 in the 4 dose cohort and SEPREHVIR® has been well-tolerated with few adverse events in any patients. An exploratory objective, to assess tumor responses by CT using modified RECIST criteria, has demonstrated disease stabilisation in 6 / 10 evaluable patients.
[0585]Pleural fluid and plasma samples h...
example 2
i-Tumor Serum IgG Response
[0630]Patient serum samples were used to probe cell extracts in order to investigate the possibility of an anti-tumor antibody response to treatment with SEPREHVIR®. Cell extracts were prepared from cell lines: MSTO-211H (mesothelioma; ATCC CRL-2081), SPC111 (mesothelioma), and HuH7 (hepatic carcinoma), and contacted with patient sera. IgG:target complexes were detected using an anti-human IgG antibody by standard Western Blotting procedures (FIG. 15).
[0631]Analysis of plasma samples indicated a strong anti-HSV IgG responses post SEPREHVIR® administration, particularly after 2 and 4 doses. Intrapleural administration of SEPREHVIR® was found to induce a novel anti-tumor IgG response against an antigen present on MSTO-211H cells but not on SPC111 or HuH7 cells (FIG. 16-18) more so in patients receiving 4 doses of Seprehvir.
[0632]Thus, SEPREHVIR® has immunotherapeutic potential capable of inducing novel anti-tumor immune responses in patients. This result is c...
example 3
t Blockade Enhances Oncolytic Herpes Virotherapy in Immunosuppressive Sarcoma Models
[0633]Most solid tumors are characterized by an immunosuppressive microenvironment, limiting the effectiveness of antitumor immunotherapeutics. Programmed cell death protein (PD)-1-mediated T cell suppression via engagement of its ligand, PD-L1, is of particular interest due to recent successes in selected adult cancers. The utility of PD1-directed therapy for pediatric cancers is unknown, especially given the paucity of mutations and thus infrequent neoantigens in many types of childhood tumors. Oncolytic virotherapy induces tumor shrinkage via a multistep process including direct tumor cell lysis, induction of cytotoxic or apoptosis-sensitizing cytokines, and induction of antitumor T cell responses. We have demonstrated that intratumoral injection of an oncolytic herpes virus induced growth delays and in some cases durable remissions in several mouse models of rhabdomyosarcoma. The effects were T c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com