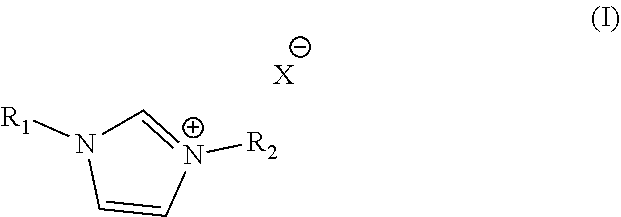
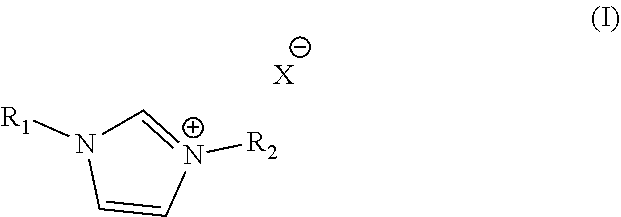
Electroless copper plating compositions and methods for electroless plating copper on substrates
a technology of electroless plating and copper, applied in the direction of liquid/solution decomposition chemical coating, metal material coating process, coating, etc., can solve the problems of bath instability, difficult control of baths, and inability to maintain substantially uniform copper deposits over long periods of time, etc., to achieve good through-hole wall coverage, increase electroless copper plating rate, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Electroless Copper Plating Rates of an Electroless Copper Plating Baths Containing 1-Benzyl-3-Methylimidazolium Chloride
[0061]Four (4) electroless copper plating baths are prepared. All four baths include the following components:
TABLE 1Bath 4ComponentBath 1Bath 2Bath 3(Control)Copper sulfate10g / L10g / L10g / L10g / LpentahydrateRochelle salts40g / L40g / L40g / L40g / LSodium hydroxide8g / L8g / L8g / L8g / LFormaldehyde4g / L4g / L4g / L4g / L2,2′-ditho-0.5ppm0.5ppm0.5ppm0.5ppmdisuccinic acid1-benzyl-3-2.5ppm10ppm20ppm—methylimidazoliumchlorideWaterTo oneTo oneTo oneTo oneliterliterliterliter
The pH of each bath is 13. Bath 4 is a control. Each bath is used to plate copper on epoxy substrates. Each epoxy substrate is first treated according to the following process prior to electroless copper plating:[0062](1) Conditioner 231 applied for 1.5 min. at 45° C.;[0063](2) Rinse with DI water for 2 min. at room temperature;[0064](3) Nitric acid pre-dip, pH=2, for 0.5 min. at room temperature;[0065](4) 100 ppm of CIRCU...
example 2
Electroless Copper Plating Rates of an Electroless Copper Plating Baths Containing 1-Benzyl-3-Methylimidazolium Chloride and Guanidine Hydrochloride
[0069]Six (6) electroless copper plating baths are prepared. The pH of each bath is 13. The baths include the components and amounts as shown in Table 3.
TABLE 3Bath 10ComponentBath 5Bath 6Bath 7Bath 8Bath 9(Control)Copper sulfate pentahydrate10g / L10g / L10g / L10g / L10g / L10g / LRochelle salts40g / L40g / L40g / L40g / L40g / L40g / LSodium hydroxide8g / L8g / L8g / L8g / L8g / L8g / LFormaldehyde4g / L4g / L4g / L4g / L4g / L4g / L2,2′-dithio-disuccinic0.5ppm0.5ppm0.5ppm0.5ppm0.5ppm0.5ppmacidGuanidine hydrochloride0.4ppm0.4ppm0.4ppm0.4ppm0.4ppm0.4ppm1-benzyl-3-methylimidazolium2.5ppm5ppm10ppm15ppm20ppm—chlorideWaterTo oneTo oneTo oneTo oneTo oneTo oneliterliterliterliterliterliter
Each bath is used to plate copper on epoxy substrates. Each epoxy substrate is treated prior to electroless copper plating as described in Example 1. Electroless copper plating is done at 34° C. for 5 mi...
example 3
Backlight Experiment with Aqueous Alkaline Electroless Cooper Compositions of the Present Invention Containing 1-Benzyl-3-Methylimidazolium Chloride
[0071]The following aqueous alkaline electroless copper compositions of the invention are prepared having the components and amounts disclosed in Table 5. Bath 11 has a pH=12.5 at room temperature as measured using a conventional pH meter available from Fisher Scientific.
TABLE 5ComponentBath 11Copper sulfate pentahydrate10g / LRochelle salts40g / LSodium hydroxide8g / LFormaldehyde4g / L2,2′-dithiosuccinic acid0.5ppmGuanidine hydrochloride0.4ppm1-benzyl-3-methylimidazolium chloride10ppmWaterOne liter
[0072]Six (6) different FR / 4 glass epoxy panels with a plurality of through-holes are provided: TUC-662, SY-1141, IT-180, 370HR, EM825 and NPGN. The panels are either four-layer or eight-layer copper-clad panels. TUC-662 is obtained from Taiwan Union Technology, and SY-1141 is obtained from Shengyi. IT-180 is obtained from ITEQ Corp., NPGN is obtaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com