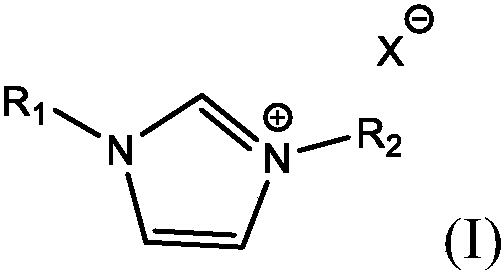
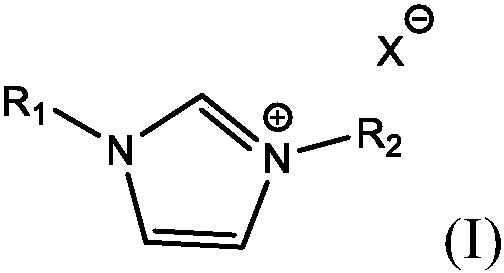
Electroless copper plating compositions and methods for electroless plating copper on substrates
A technology for electroless copper plating and composition, applied in the field of electroless copper plating compositions and for electroless copper plating on substrates, can solve problems such as reducing the stability of electroless copper baths, achieve lower operating costs, lower consumption, The effect of good through-hole wall coverage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0065] Electroless Copper Plating Rates in Electroless Copper Baths Containing 1-Benzyl-3-methylimidazolium Chloride
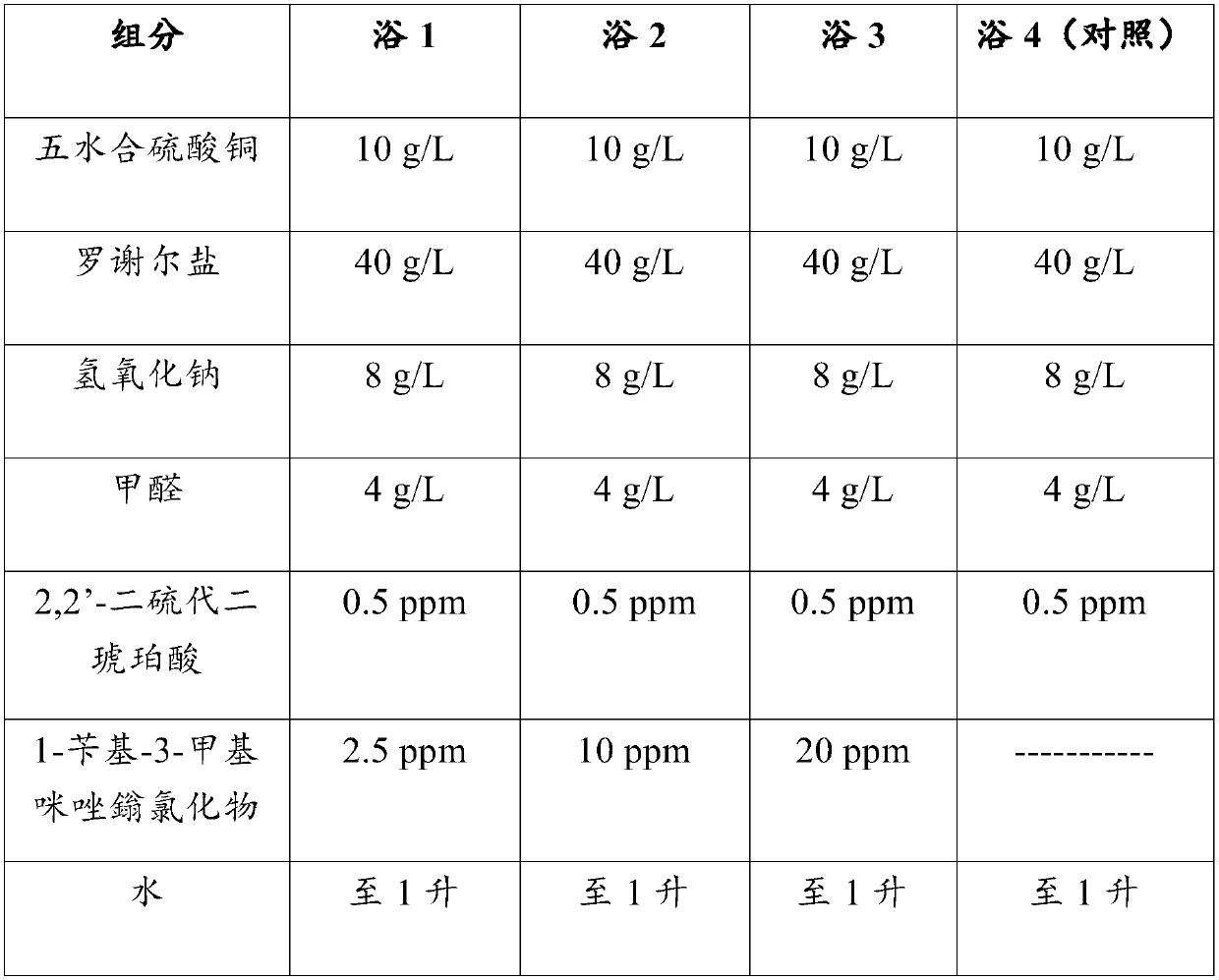
[0066] Four (4) electroless copper plating baths were prepared. All four baths include the following components:
[0067] Table 1
[0068]
[0069] The pH of each bath was 13. Bath 4 was the control. Each bath is used to plate copper on epoxy substrates. Prior to electroless copper plating, each epoxy substrate was first treated according to the following process:
[0070] (1) Apply conditioner 231 at 45°C for 1.5 minutes;
[0071] (2) Rinse with deionized water for 2 minutes at room temperature;
[0072] (3) Apply nitric acid prepreg pH=2 at room temperature for 0.5 minutes;
[0073] (4) Apply 100ppm of CIRCUPOSIT at 40°C TM 6530 ionic palladium catalyst for 1 minute;
[0074] (5) Rinse with deionized water for 1 minute at room temperature;
[0075] (6) At 30° C., apply 5 g / L boric acid and 0.6 g / L dimethylamine borane aqueous solution for 1 min...
example 2
[0082] Electroless Copper Plating Rates of Electroless Copper Plating Baths Containing 1-Benzyl-3-methylimidazolium Chloride and Guanidine Hydrochloride
[0083] Six (6) electroless copper plating baths were prepared. The pH of each bath was 13. The baths included the components and amounts shown in Table 3.
[0084] table 3
[0085]
[0086] Each bath is used to plate copper on epoxy substrates. Each epoxy substrate was treated as described in Example 1 prior to electroless copper plating. Electroless copper plating was performed at 34°C for 5 minutes. Plating rate was determined by the same procedure as described in Example 1. Plating rates for each bath are shown in Table 4.
[0087] Table 4
[0088] bath Plating speed 5 0.74μm / 5min. 6 0.67μm / 5min. 7 0.79μm / 5min. 8 0.63μm / 5min. 9 0.7μm / 5min. 10 (control) 0.5μm / 5min.
[0089] Inclusion of 1-benzyl-3-methylimidazolium chloride in the electroless copper plating bat...
example 3
[0092] Backlight Experiments of Aqueous Alkaline Electroless Copper Compositions of the Invention Containing 1-Benzyl-3-Methylimidazolium Chloride
[0093] The following aqueous alkaline electroless copper compositions of the present invention having the components and amounts disclosed in Table 5 were prepared. The pH of bath 11 at room temperature = 12.5 as measured using a conventional pH meter available from Fisher Scientific.
[0094] table 5
[0095] components bath 11 copper sulfate pentahydrate 10g / L rochelle salt 40g / L sodium hydroxide 8g / L formaldehyde 4g / L 2,2'-dithiosuccinic acid 0.5ppm Guanidine hydrochloride 0.4ppm 1-Benzyl-3-methylimidazolium chloride 10ppm water 1 littre
[0096] Six (6) different FR / 4 glass epoxy boards with multiple through holes are available: TUC-662, SY-1141, IT-180, 370HR, EM825 and NPGN. Boards are four- or eight-layer copper-clad boards. TUC-662 was obtained from...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com