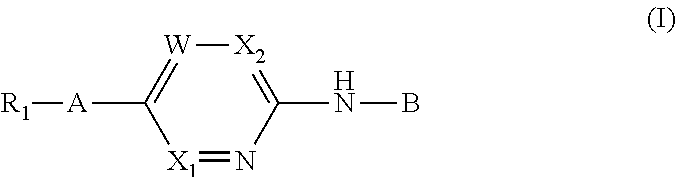
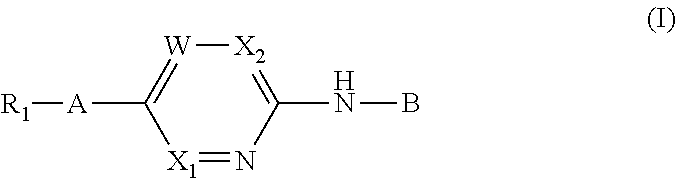
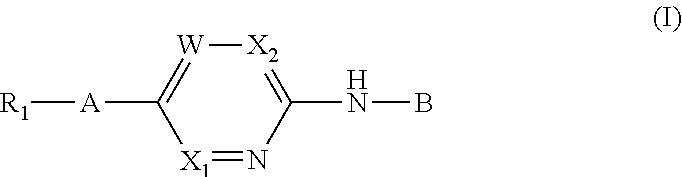
Aminopyrimidine derivatives as phosphatidylinositol phosphate kinase inhibitors
a technology of phosphatidylinositol and derivatives, which is applied in the direction of antineoplastic agents, organic chemistry, drug compositions, etc., can solve the problems of no known therapeutic agents which effectively inhibit the synthesis of pip5kii-beta, and achieve the effect of improving the efficacy and safety profil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
14: N-{5-[2-(3,3-difluoropyrrolidin-1-yl)ethoxy]pyridin-3-yl}-4-methoxy-5-[4-(4-methylpyridazin-3-yl)phenyl]pyrimidin-2-amine
[0434]
Example 2: Compound 8: (6S)-12-({5-[4-(4-methyl-4H-1,2,4-triazol-3-yl)phenyl]-4-(prop-1-en-2-yl)pyridin-2-yl}amino)-8-oxa-2,10-diazatricyclo[7.4.0.02,6]trideca-1(13),9,11-trien-3-one
[0435]
Example 3: Compound 14: (6S)-12-({5-[4-(2-oxopyrrolidin-1-yl)phenyl]-4-(trifluoromethyl)pyrimidin-2-yl}amino)8-oxa-2,10-diazatricycloylo[7.4.0.02,6]trideca-1(9),19,12-trien-3-one
[0436]
CompoundNo.StructureCompound Name 12-[(2-{[(6S)-3-oxo-8-oxa-2,10- diazatricyclo[7.4.0.02,6]trideca- 1(9),10,12-trien-12-yl]amino}-5-[4- (2-oxopyrrolidin-1- yl)phenyl]pyrimidin-4- yl)amino]acetic acid 2(6S)-12-[(4-{methyl[(3S)-oxolan-3- yl]amino}-5-[4-(2-oxopyrrolidin-1- yl)phenyl]pyrimidin-2-yl)amino]-8- oxa-2,10- diazatricyclo[7.4.0.02,6]trideca- 1(13),9,11-trien-3-one 3(6S)-12-({4-[methyl(oxolan-3- yl)amino]-5-[4-(2-oxopyrrolidin-1- yl)phenyl]pyrimidin-2-yl}amino)-8- oxa-2,10- diazatricy...
example 4
iochemical Assay
[0437]Dilution series of the compounds were prepared in DMSO at 100 times the final assay concentration (n1=n0 / 3 in 10 points). The compounds were further diluted to three times the assay concentration in assay buffer (20 mM MOPS pH 7.2, 25 mM magnesium chloride, 0.005% Tween 20). 6 μL of the diluted compounds were added to a 384 well assay plate followed by 9 μL of a mix consisting of 4 nM PIP4K2A (full length protein, SignalChem) and 100 M P(5)P diC8 (Tebu-Bio). Enzyme and compounds were pre-incubated at room temperature for 15 minutes.
[0438]Then 3 μL of a solution containing 60 M ATP (Promega) in assay buffer was added to the wells containing compound and enzyme and mixing was performed by pipetting several times. The reaction was incubated at room temperature for 1h. Then 18 μL of ADP-Glo™ Reagent (Promega) was added to stop the kinase reaction and deplete the unconsumed ATP, mixing was performed by pipetting several times. The plate was incubated at room tempera...
example 5
tocol—PIP4KtypeIIA
[0440]GST tagged PIP4KtypeIIA and B enzymes were overexpressed in E. Coli and purified to >80% homogeneity. Phosphatidyl inositol-5-phosphate (PI5P, Cat. #850152, Avanti Polar Lipids Inc.) was used as the lipid substrate and phosphatidyl ethanolamine (DOPE 18:1, Cat. #850725, Avanti Polar Lipids Inc.) was used as the carrier lipid for assays. Ultrapure ATP and GTP was purchased from Bellbrooke Labs. ADP Glo reagents were obtained from Promega. Transcreener FI reagent was obtained from Bellbrooke labs.
Buffers:
[0441]1. HEPES buffer mix: 200 mM HEPES pH 7.4, 50 mM MgCl2, 0.05% v / v triton X 100
2. HNE buffer: 20 mM HEPES, pH 7.4, 100 mM NaCl, 0.5 mM EGTA
3. H:E buffer: 30 mM HEPES, pH 7.4, 1 mM EGTA
[0442]Enzyme preparation: GST-tagged PIP4KtypeIIA (5 μL, 1.43 mg / mL) was diluted (1:10) to 50 μL using HNE buffer. From the 1:10 diluted stock, a 6.4 μL aliquot was diluted further to 5 mL using HNE buffer to yield 5× enzyme stock (2.5 nM).
GST-tagged PIP4KtypeIIB (3.4 μL, 2.77...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com